| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen phosphorylase, muscle form |
|---|
| Ligand | BDBM50263771 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_535893 (CHEMBL983534) |
|---|
| Ki | 4600±n/a nM |
|---|
| Citation |  Somsák, L; Nagy, V; Vidal, S; Czifrák, K; Berzsényi, E; Praly, JP Novel design principle validated: glucopyranosylidene-spiro-oxathiazole as new nanomolar inhibitor of glycogen phosphorylase, potential antidiabetic agent. Bioorg Med Chem Lett18:5680-3 (2008) [PubMed] Article Somsák, L; Nagy, V; Vidal, S; Czifrák, K; Berzsényi, E; Praly, JP Novel design principle validated: glucopyranosylidene-spiro-oxathiazole as new nanomolar inhibitor of glycogen phosphorylase, potential antidiabetic agent. Bioorg Med Chem Lett18:5680-3 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen phosphorylase, muscle form |
|---|
| Name: | Glycogen phosphorylase, muscle form |
|---|
| Synonyms: | Glycogen Phosphorylase (PYGM) | Glycogen phosphorylase a (RMGPa) | Glycogen phosphorylase, muscle form | Myophosphorylase | PYGM | PYGM_RABIT |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 97296.32 |
|---|
| Organism: | Oryctolagus cuniculus (rabbit) |
|---|
| Description: | Phosphorylation of Ser-15 converts phosphorylase B (unphosphorylated) to phosphorylase A. |
|---|
| Residue: | 843 |
|---|
| Sequence: | MSRPLSDQEKRKQISVRGLAGVENVTELKKNFNRHLHFTLVKDRNVATPRDYYFALAHTV
RDHLVGRWIRTQQHYYEKDPKRIYYLSLEFYMGRTLQNTMVNLALENACDEATYQLGLDM
EELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEFGIFNQKICGGWQMEEA
DDWLRYGNPWEKARPEFTLPVHFYGRVEHTSQGAKWVDTQVVLAMPYDTPVPGYRNNVVN
TMRLWSAKAPNDFNLKDFNVGGYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFV
VAATLQDIIRRFKSSKFGCRDPVRTNFDAFPDKVAIQLNDTHPSLAIPELMRVLVDLERL
DWDKAWEVTVKTCAYTNHTVLPEALERWPVHLLETLLPRHLQIIYEINQRFLNRVAAAFP
GDVDRLRRMSLVEEGAVKRINMAHLCIAGSHAVNGVARIHSEILKKTIFKDFYELEPHKF
QNKTNGITPRRWLVLCNPGLAEIIAERIGEEYISDLDQLRKLLSYVDDEAFIRDVAKVKQ
ENKLKFAAYLEREYKVHINPNSLFDVQVKRIHEYKRQLLNCLHVITLYNRIKKEPNKFVV
PRTVMIGGKAAPGYHMAKMIIKLITAIGDVVNHDPVVGDRLRVIFLENYRVSLAEKVIPA
ADLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENFFIFGMRVEDVD
RLDQRGYNAQEYYDRIPELRQIIEQLSSGFFSPKQPDLFKDIVNMLMHHDRFKVFADYEE
YVKCQERVSALYKNPREWTRMVIRNIATSGKFSSDRTIAQYAREIWGVEPSRQRLPAPDE
KIP
|
|
|
|---|
| BDBM50263771 |
|---|
| n/a |
|---|
| Name | BDBM50263771 |
|---|
| Synonyms: | 1-benzoyl-3-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-yl)urea | CHEMBL489799 | N-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-ylcarbamoyl)benzamide | N-BENZOYL-N'-BETA-D-GLUCOPYRANOSYL UREA |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H18N2O7 |
|---|
| Mol. Mass. | 326.3019 |
|---|
| SMILES | OC[C@H]1O[C@@H](NC(=O)NC(=O)c2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| |
|---|
| Structure | 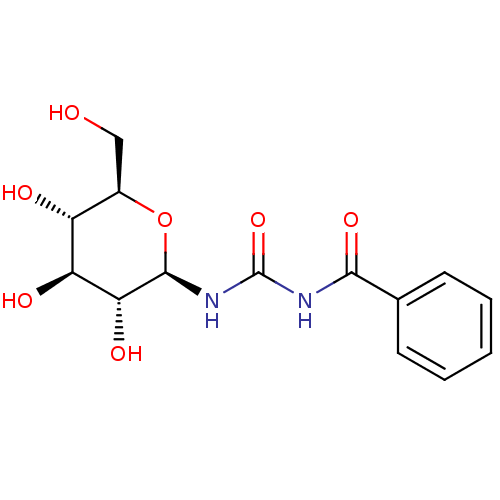
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Somsák, L; Nagy, V; Vidal, S; Czifrák, K; Berzsényi, E; Praly, JP Novel design principle validated: glucopyranosylidene-spiro-oxathiazole as new nanomolar inhibitor of glycogen phosphorylase, potential antidiabetic agent. Bioorg Med Chem Lett18:5680-3 (2008) [PubMed] Article
Somsák, L; Nagy, V; Vidal, S; Czifrák, K; Berzsényi, E; Praly, JP Novel design principle validated: glucopyranosylidene-spiro-oxathiazole as new nanomolar inhibitor of glycogen phosphorylase, potential antidiabetic agent. Bioorg Med Chem Lett18:5680-3 (2008) [PubMed] Article