| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50252735 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_539794 (CHEMBL1035787) |
|---|
| IC50 | 1800±n/a nM |
|---|
| Citation |  Pettus, LH; Xu, S; Cao, GQ; Chakrabarti, PP; Rzasa, RM; Sham, K; Wurz, RP; Zhang, D; Middleton, S; Henkle, B; Plant, MH; Saris, CJ; Sherman, L; Wong, LM; Powers, DA; Tudor, Y; Yu, V; Lee, MR; Syed, R; Hsieh, F; Tasker, AS 3-amino-7-phthalazinylbenzoisoxazoles as a novel class of potent, selective, and orally available inhibitors of p38alpha mitogen-activated protein kinase. J Med Chem51:6280-92 (2008) [PubMed] Article Pettus, LH; Xu, S; Cao, GQ; Chakrabarti, PP; Rzasa, RM; Sham, K; Wurz, RP; Zhang, D; Middleton, S; Henkle, B; Plant, MH; Saris, CJ; Sherman, L; Wong, LM; Powers, DA; Tudor, Y; Yu, V; Lee, MR; Syed, R; Hsieh, F; Tasker, AS 3-amino-7-phthalazinylbenzoisoxazoles as a novel class of potent, selective, and orally available inhibitors of p38alpha mitogen-activated protein kinase. J Med Chem51:6280-92 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50252735 |
|---|
| n/a |
|---|
| Name | BDBM50252735 |
|---|
| Synonyms: | CHEMBL495084 | N-Cyclopropyl-6-methyl-7-(1-o-tolylphthalazin-6-yl)benzo[d]-isoxazol-3-amine |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H22N4O |
|---|
| Mol. Mass. | 406.4791 |
|---|
| SMILES | Cc1ccccc1-c1nncc2cc(ccc12)-c1c(C)ccc2c(NC3CC3)noc12 |
|---|
| Structure | 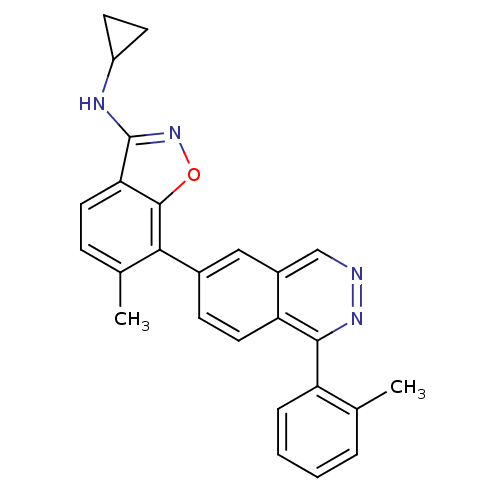
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pettus, LH; Xu, S; Cao, GQ; Chakrabarti, PP; Rzasa, RM; Sham, K; Wurz, RP; Zhang, D; Middleton, S; Henkle, B; Plant, MH; Saris, CJ; Sherman, L; Wong, LM; Powers, DA; Tudor, Y; Yu, V; Lee, MR; Syed, R; Hsieh, F; Tasker, AS 3-amino-7-phthalazinylbenzoisoxazoles as a novel class of potent, selective, and orally available inhibitors of p38alpha mitogen-activated protein kinase. J Med Chem51:6280-92 (2008) [PubMed] Article
Pettus, LH; Xu, S; Cao, GQ; Chakrabarti, PP; Rzasa, RM; Sham, K; Wurz, RP; Zhang, D; Middleton, S; Henkle, B; Plant, MH; Saris, CJ; Sherman, L; Wong, LM; Powers, DA; Tudor, Y; Yu, V; Lee, MR; Syed, R; Hsieh, F; Tasker, AS 3-amino-7-phthalazinylbenzoisoxazoles as a novel class of potent, selective, and orally available inhibitors of p38alpha mitogen-activated protein kinase. J Med Chem51:6280-92 (2008) [PubMed] Article