| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Mitogen-activated protein kinase 8 |
|---|
| Ligand | BDBM50277906 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_501722 (CHEMBL985050) |
|---|
| Ki | 530±n/a nM |
|---|
| Citation |  Humphries, PS; Lafontaine, JA; Agree, CS; Alexander, D; Chen, P; Do, QQ; Li, LY; Lunney, EA; Rajapakse, RJ; Siegel, K; Timofeevski, SL; Wang, T; Wilhite, DM Synthesis and SAR of 4-substituted-2-aminopyrimidines as novel c-Jun N-terminal kinase (JNK) inhibitors. Bioorg Med Chem Lett19:2099-102 (2009) [PubMed] Article Humphries, PS; Lafontaine, JA; Agree, CS; Alexander, D; Chen, P; Do, QQ; Li, LY; Lunney, EA; Rajapakse, RJ; Siegel, K; Timofeevski, SL; Wang, T; Wilhite, DM Synthesis and SAR of 4-substituted-2-aminopyrimidines as novel c-Jun N-terminal kinase (JNK) inhibitors. Bioorg Med Chem Lett19:2099-102 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Mitogen-activated protein kinase 8 |
|---|
| Name: | Mitogen-activated protein kinase 8 |
|---|
| Synonyms: | JNK-46 | JNK1 | JNK1-alpha-1 | MAPK8 | MK08_HUMAN | Mitogen-Activated Protein Kinase 8 (JNK1) | PRKM8 | SAPK1 | SAPK1C | Stress-activated protein kinase JNK1 | c-Jun N-terminal kinase 1 | c-Jun N-terminal kinase 1 (JNK1) | c-Jun N-terminal kinase 1(JNK1) | c-Jun N-terminal kinase 2 (JNK2) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48297.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | JNK-1 was purchased from Upstate Cell Signaling Solutions (formerly Upstate Biotechnology). |
|---|
| Residue: | 427 |
|---|
| Sequence: | MSRSKRDNNFYSVEIGDSTFTVLKRYQNLKPIGSGAQGIVCAAYDAILERNVAIKKLSRP
FQNQTHAKRAYRELVLMKCVNHKNIIGLLNVFTPQKSLEEFQDVYIVMELMDANLCQVIQ
MELDHERMSYLLYQMLCGIKHLHSAGIIHRDLKPSNIVVKSDCTLKILDFGLARTAGTSF
MMTPYVVTRYYRAPEVILGMGYKENVDLWSVGCIMGEMVCHKILFPGRDYIDQWNKVIEQ
LGTPCPEFMKKLQPTVRTYVENRPKYAGYSFEKLFPDVLFPADSEHNKLKASQARDLLSK
MLVIDASKRISVDEALQHPYINVWYDPSEAEAPPPKIPDKQLDEREHTIEEWKELIYKEV
MDLEERTKNGVIRGQPSPLGAAVINGSQHPSSSSSVNDVSSMSTDPTLASDTDSSLEAAA
GPLGCCR
|
|
|
|---|
| BDBM50277906 |
|---|
| n/a |
|---|
| Name | BDBM50277906 |
|---|
| Synonyms: | CHEMBL484326 | N-isopropyl-4-(3-(piperidin-3-yl)-1H-pyrazol-4-yl)pyrimidin-2-amine |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H22N6 |
|---|
| Mol. Mass. | 286.3754 |
|---|
| SMILES | CC(C)Nc1nccc(n1)-c1cn[nH]c1C1CCCNC1 |
|---|
| Structure | 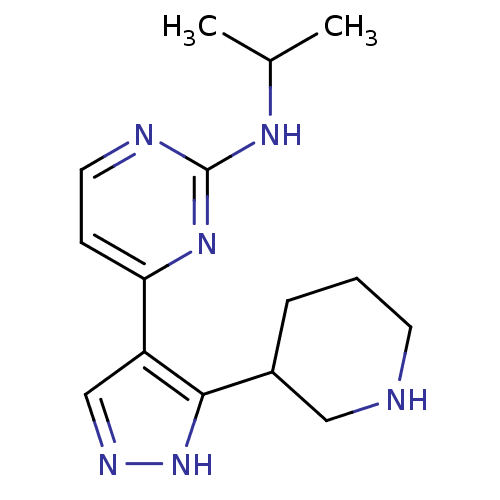
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Humphries, PS; Lafontaine, JA; Agree, CS; Alexander, D; Chen, P; Do, QQ; Li, LY; Lunney, EA; Rajapakse, RJ; Siegel, K; Timofeevski, SL; Wang, T; Wilhite, DM Synthesis and SAR of 4-substituted-2-aminopyrimidines as novel c-Jun N-terminal kinase (JNK) inhibitors. Bioorg Med Chem Lett19:2099-102 (2009) [PubMed] Article
Humphries, PS; Lafontaine, JA; Agree, CS; Alexander, D; Chen, P; Do, QQ; Li, LY; Lunney, EA; Rajapakse, RJ; Siegel, K; Timofeevski, SL; Wang, T; Wilhite, DM Synthesis and SAR of 4-substituted-2-aminopyrimidines as novel c-Jun N-terminal kinase (JNK) inhibitors. Bioorg Med Chem Lett19:2099-102 (2009) [PubMed] Article