| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50050632 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_197421 (CHEMBL799810) |
|---|
| IC50 | 156±n/a nM |
|---|
| Citation |  Terrett, NT; Bojanic, D; Merson, JR; Stephenson, PT Imidazo[2′-3′-:6,5]dipyrido[3,2-b:2′,3′-e]-1,4-diazepines: non-nucleoside HIV-1 reverse transcriptase inhibitors with greater enzyme affinity than nevirapine Bioorg Med Chem Lett2:1745-1750 (1992) Article Terrett, NT; Bojanic, D; Merson, JR; Stephenson, PT Imidazo[2′-3′-:6,5]dipyrido[3,2-b:2′,3′-e]-1,4-diazepines: non-nucleoside HIV-1 reverse transcriptase inhibitors with greater enzyme affinity than nevirapine Bioorg Med Chem Lett2:1745-1750 (1992) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50050632 |
|---|
| n/a |
|---|
| Name | BDBM50050632 |
|---|
| Synonyms: | 8-ETHYL-6-METHOXY-3-METHYL-8H-1,3A,7,8,9-PENTAAZA-DIBENZO[E,H]AZULENE | CHEMBL42906 | UK-129485 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H17N5O |
|---|
| Mol. Mass. | 307.3498 |
|---|
| SMILES | CCN1c2ncccc2-c2ncc(C)n2-c2ccc(OC)nc12 |
|---|
| Structure | 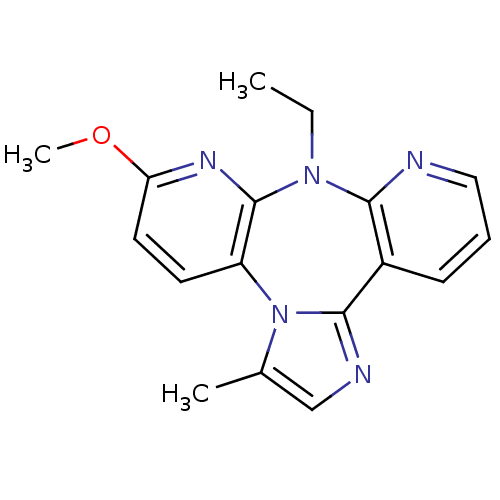
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Terrett, NT; Bojanic, D; Merson, JR; Stephenson, PT Imidazo[2′-3′-:6,5]dipyrido[3,2-b:2′,3′-e]-1,4-diazepines: non-nucleoside HIV-1 reverse transcriptase inhibitors with greater enzyme affinity than nevirapine Bioorg Med Chem Lett2:1745-1750 (1992) Article
Terrett, NT; Bojanic, D; Merson, JR; Stephenson, PT Imidazo[2′-3′-:6,5]dipyrido[3,2-b:2′,3′-e]-1,4-diazepines: non-nucleoside HIV-1 reverse transcriptase inhibitors with greater enzyme affinity than nevirapine Bioorg Med Chem Lett2:1745-1750 (1992) Article