| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Type-1 angiotensin II receptor B |
|---|
| Ligand | BDBM50030727 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_34824 (CHEMBL648758) |
|---|
| IC50 | 0.31±n/a nM |
|---|
| Citation |  Chang, LL; Ashton, WT; Flanagan, KL; Naylor, EM; Chakravarty, PK; Patchett, AA; Greenlee, WJ; Bendesky, RJ; Chen, TB; Faust, KA; Kling, PJ; Schaffer, LW; Schorn, TW; Zingaro, GJ; Chang, RS; Lotti, VJ; Kivlighn, SD; Siegl, PK Triazolinones as nonpeptide angiotensin II antagonists. 2. discovery of a potent and orally active triazolinone acylsulfonamide Bioorg Med Chem Lett4:115-120 (1994) Article Chang, LL; Ashton, WT; Flanagan, KL; Naylor, EM; Chakravarty, PK; Patchett, AA; Greenlee, WJ; Bendesky, RJ; Chen, TB; Faust, KA; Kling, PJ; Schaffer, LW; Schorn, TW; Zingaro, GJ; Chang, RS; Lotti, VJ; Kivlighn, SD; Siegl, PK Triazolinones as nonpeptide angiotensin II antagonists. 2. discovery of a potent and orally active triazolinone acylsulfonamide Bioorg Med Chem Lett4:115-120 (1994) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Type-1 angiotensin II receptor B |
|---|
| Name: | Type-1 angiotensin II receptor B |
|---|
| Synonyms: | AGTRB_RAT | AT3 | Agtr1 | Agtr1b | Angiotensin II AT1B | Angiotensin II receptor (AT-1) type-1 | Angiotensin II type 1b (AT-1b) receptor | At1b | Type-1B angiotensin II receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 40929.44 |
|---|
| Organism: | RAT |
|---|
| Description: | Angiotensin II AT1B 0 RAT::P29089 |
|---|
| Residue: | 359 |
|---|
| Sequence: | MTLNSSTEDGIKRIQDDCPKAGRHNYIFVMIPTLYSIIFVVGIFGNSLVVIVIYFYMKLK
TVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNHLCKIASASVSFNLYASVFLLT
CLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLMAGLASLPAVIYRNVYFIENTNITVC
AFHYESQNSTLPIGLGLTKNILGFVFPFLIILTSYTLIWKALKKAYKIQKNTPRNDDIFR
IIMAIVLFFFFSWVPHQIFTFLDVLIQLGIIRDCEIADIVDTAMPITICIAYFNNCLNPL
FYGFLGKKFKKYFLQLLKYIPPTAKSHAGLSTKMSTLSYRPSDNMSSSAKKSASFFEVE
|
|
|
|---|
| BDBM50030727 |
|---|
| n/a |
|---|
| Name | BDBM50030727 |
|---|
| Synonyms: | 4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5-dihydro-[1,2,4]triazol-4-ylmethyl]-biphenyl-2-sulfonic acid benzoylamide | CHEMBL279629 | CHEMBL97539 | L-159,913 | L-159913 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H29F3N4O4S |
|---|
| Mol. Mass. | 634.668 |
|---|
| SMILES | CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 |
|---|
| Structure | 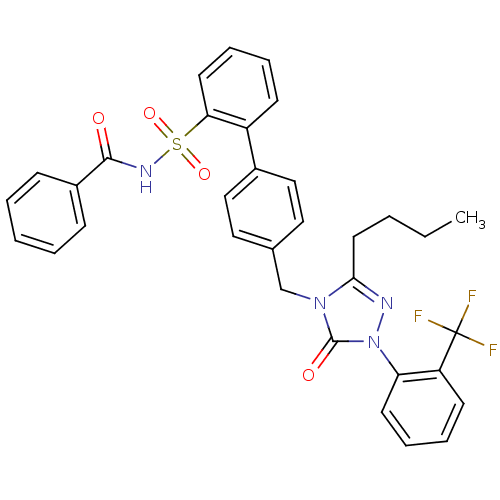
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chang, LL; Ashton, WT; Flanagan, KL; Naylor, EM; Chakravarty, PK; Patchett, AA; Greenlee, WJ; Bendesky, RJ; Chen, TB; Faust, KA; Kling, PJ; Schaffer, LW; Schorn, TW; Zingaro, GJ; Chang, RS; Lotti, VJ; Kivlighn, SD; Siegl, PK Triazolinones as nonpeptide angiotensin II antagonists. 2. discovery of a potent and orally active triazolinone acylsulfonamide Bioorg Med Chem Lett4:115-120 (1994) Article
Chang, LL; Ashton, WT; Flanagan, KL; Naylor, EM; Chakravarty, PK; Patchett, AA; Greenlee, WJ; Bendesky, RJ; Chen, TB; Faust, KA; Kling, PJ; Schaffer, LW; Schorn, TW; Zingaro, GJ; Chang, RS; Lotti, VJ; Kivlighn, SD; Siegl, PK Triazolinones as nonpeptide angiotensin II antagonists. 2. discovery of a potent and orally active triazolinone acylsulfonamide Bioorg Med Chem Lett4:115-120 (1994) Article