| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM50285522 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_1234 |
|---|
| IC50 | 20±n/a nM |
|---|
| Citation |  Macor, JE; Blank, DH; Desai, K; Fox, CB; Koe, BK; Lebel, LA; Post, RJ; Schmidt, AW; Schulz, DW; Seymour, PA 5-cyano-1-[3-(N-methylpyrrolidin-2R-ylmethyl)indol-5-yl] benzimidazole (CP-161,242): A potent, centrally active 5-HT1D receptor agonist and benzodiazepine partial agonist Bioorg Med Chem Lett5:2391-2396 (1995) Article Macor, JE; Blank, DH; Desai, K; Fox, CB; Koe, BK; Lebel, LA; Post, RJ; Schmidt, AW; Schulz, DW; Seymour, PA 5-cyano-1-[3-(N-methylpyrrolidin-2R-ylmethyl)indol-5-yl] benzimidazole (CP-161,242): A potent, centrally active 5-HT1D receptor agonist and benzodiazepine partial agonist Bioorg Med Chem Lett5:2391-2396 (1995) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT-1A | 5-HT1 | 5-HT1A | 5-Hydroxytryptamine receptor 1A (5-HT1A) | 5-hydroxytryptamine receptor 1A (5HT1A) | 5HT1A_RAT | 5ht1a | G-21 | Htr1a | Serotonin 1 (5-HT1) receptor | Serotonin 1a (5-HT1a) receptor/Adrenergic receptor alpha-1 | Serotonin receptor 1A |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 46445.29 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Binding assays were performed using rat hippocampal membranes. |
|---|
| Residue: | 422 |
|---|
| Sequence: | MDVFSFGQGNNTTASQEPFGTGGNVTSISDVTFSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVRKVEKKGAGT
SLGTSSAPPPKKSLNGQPGSGDWRRCAENRAVGTPCTNGAVRQGDDEATLEVIEVHRVGN
SKEHLPLPSESGSNSYAPACLERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPALLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
RR
|
|
|
|---|
| BDBM50285522 |
|---|
| n/a |
|---|
| Name | BDBM50285522 |
|---|
| Synonyms: | 1-[3-((R)-1-Methyl-pyrrolidin-2-ylmethyl)-1H-indol-5-yl]-1H-benzoimidazole-5-carbonitrile | CHEMBL75642 | CP-161242 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H21N5 |
|---|
| Mol. Mass. | 355.4356 |
|---|
| SMILES | CN1CCC[C@@H]1Cc1c[nH]c2ccc(cc12)-n1cnc2cc(ccc12)C#N |
|---|
| Structure | 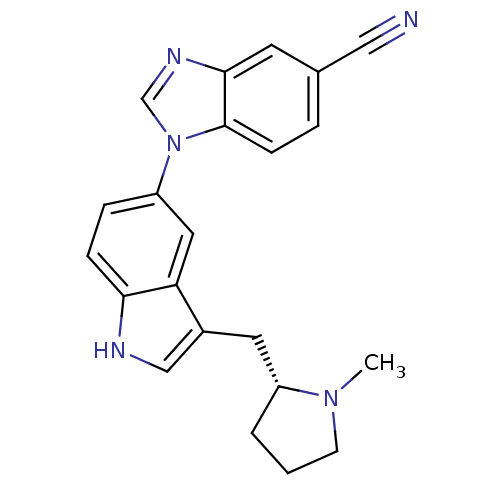
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Macor, JE; Blank, DH; Desai, K; Fox, CB; Koe, BK; Lebel, LA; Post, RJ; Schmidt, AW; Schulz, DW; Seymour, PA 5-cyano-1-[3-(N-methylpyrrolidin-2R-ylmethyl)indol-5-yl] benzimidazole (CP-161,242): A potent, centrally active 5-HT1D receptor agonist and benzodiazepine partial agonist Bioorg Med Chem Lett5:2391-2396 (1995) Article
Macor, JE; Blank, DH; Desai, K; Fox, CB; Koe, BK; Lebel, LA; Post, RJ; Schmidt, AW; Schulz, DW; Seymour, PA 5-cyano-1-[3-(N-methylpyrrolidin-2R-ylmethyl)indol-5-yl] benzimidazole (CP-161,242): A potent, centrally active 5-HT1D receptor agonist and benzodiazepine partial agonist Bioorg Med Chem Lett5:2391-2396 (1995) Article