| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50056101 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_50042 |
|---|
| IC50 | 146±n/a nM |
|---|
| Citation |  Semple, G; Ryder, H; Kendrick, DA; Szelke, M; Ohta, M; Satoh, M; Nishida, A; Akuzawa, S; Miyata, K Synthesis and biological activity of 1-alkylcarbonylmethyl analogues of YM022 Bioorg Med Chem Lett6:51-54 (1996) Article Semple, G; Ryder, H; Kendrick, DA; Szelke, M; Ohta, M; Satoh, M; Nishida, A; Akuzawa, S; Miyata, K Synthesis and biological activity of 1-alkylcarbonylmethyl analogues of YM022 Bioorg Med Chem Lett6:51-54 (1996) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49676.37 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholecystokinin central 0 RAT::P30551 |
|---|
| Residue: | 444 |
|---|
| Sequence: | MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQI
LLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLK
DFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAAT
WCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVM
VVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQL
SSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAE
KHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEE
DGRTIRALLSRYSYSHMSTSAPPP
|
|
|
|---|
| BDBM50056101 |
|---|
| n/a |
|---|
| Name | BDBM50056101 |
|---|
| Synonyms: | 1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl]-3-m-tolyl-urea | CHEMBL115121 | YM022 | YM022 Racemate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C32H28N4O3 |
|---|
| Mol. Mass. | 516.5897 |
|---|
| SMILES | Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| |
|---|
| Structure | 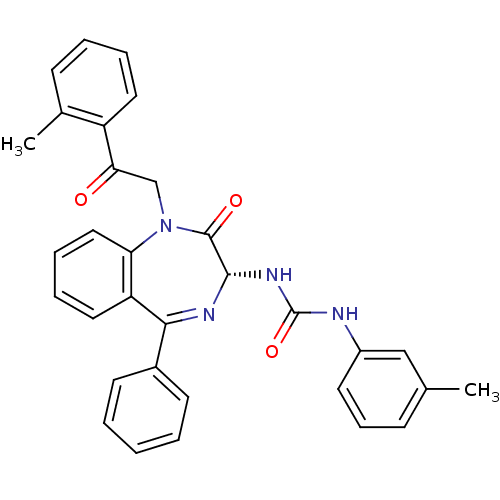
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Semple, G; Ryder, H; Kendrick, DA; Szelke, M; Ohta, M; Satoh, M; Nishida, A; Akuzawa, S; Miyata, K Synthesis and biological activity of 1-alkylcarbonylmethyl analogues of YM022 Bioorg Med Chem Lett6:51-54 (1996) Article
Semple, G; Ryder, H; Kendrick, DA; Szelke, M; Ohta, M; Satoh, M; Nishida, A; Akuzawa, S; Miyata, K Synthesis and biological activity of 1-alkylcarbonylmethyl analogues of YM022 Bioorg Med Chem Lett6:51-54 (1996) Article