| Reaction Details |
|---|
 | Report a problem with these data |
| Target | GTPase HRas |
|---|
| Ligand | BDBM50287949 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_143166 |
|---|
| IC50 | 14000±n/a nM |
|---|
| Citation |  Wolin, R; Wang, D; Kelly, J; Afonso, A; James, L; Kirschmeier, P; McPhail, AT Synthesis and evaluation of pyrazolo[3,4-b]quinoline ribofuranosides and their derivatives as inhibitors of oncogenic Ras Bioorg Med Chem Lett6:195-200 (1996) Article Wolin, R; Wang, D; Kelly, J; Afonso, A; James, L; Kirschmeier, P; McPhail, AT Synthesis and evaluation of pyrazolo[3,4-b]quinoline ribofuranosides and their derivatives as inhibitors of oncogenic Ras Bioorg Med Chem Lett6:195-200 (1996) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| GTPase HRas |
|---|
| Name: | GTPase HRas |
|---|
| Synonyms: | GTPase HRas, N-terminally processed | H-Ras | H-Ras-1 | HRAS | HRAS1 | Ha-Ras | His6-Ha-Ras-CVLS | RASH_HUMAN | Transforming protein p21 | Transforming protein p21/H-Ras-1 | Wild-type Ha-Ras | c-H-ras | p21ras |
|---|
| Type: | Other Protein Type |
|---|
| Mol. Mass.: | 21293.37 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P01112 |
|---|
| Residue: | 189 |
|---|
| Sequence: | MTEYKLVVVGAGGVGKSALTIQLIQNHFVDEYDPTIEDSYRKQVVIDGETCLLDILDTAG
QEEYSAMRDQYMRTGEGFLCVFAINNTKSFEDIHQYREQIKRVKDSDDVPMVLVGNKCDL
AARTVESRQAQDLARSYGIPYIETSAKTRQGVEDAFYTLVREIRQHKLRKLNPPDESGPG
CMSCKCVLS
|
|
|
|---|
| BDBM50287949 |
|---|
| n/a |
|---|
| Name | BDBM50287949 |
|---|
| Synonyms: | 1-[(2R,6S)-4-Benzenesulfonyl-6-(tert-butyl-dimethyl-silanyloxymethyl)-morpholin-2-yl]-4-chloro-6-methoxy-3-methyl-1H-pyrazolo[3,4-b]quinoline | CHEMBL432297 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H37ClN4O5SSi |
|---|
| Mol. Mass. | 617.231 |
|---|
| SMILES | COc1ccc2nc3n(nc(C)c3c(Cl)c2c1)[C@H]1CN(C[C@@H](CO[Si](C)(C)C(C)(C)C)O1)S(=O)(=O)c1ccccc1 |
|---|
| Structure | 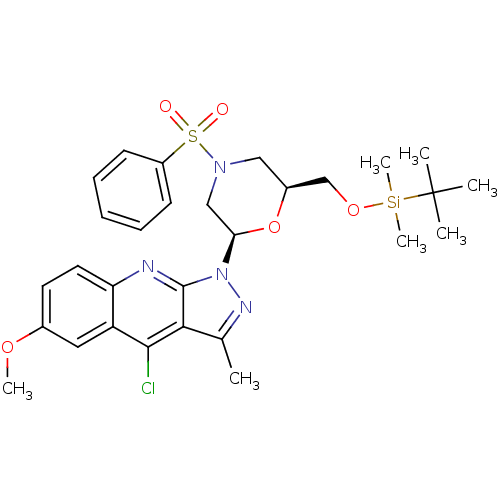
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wolin, R; Wang, D; Kelly, J; Afonso, A; James, L; Kirschmeier, P; McPhail, AT Synthesis and evaluation of pyrazolo[3,4-b]quinoline ribofuranosides and their derivatives as inhibitors of oncogenic Ras Bioorg Med Chem Lett6:195-200 (1996) Article
Wolin, R; Wang, D; Kelly, J; Afonso, A; James, L; Kirschmeier, P; McPhail, AT Synthesis and evaluation of pyrazolo[3,4-b]quinoline ribofuranosides and their derivatives as inhibitors of oncogenic Ras Bioorg Med Chem Lett6:195-200 (1996) Article