| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein phosphatase F |
|---|
| Ligand | BDBM50296378 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_580554 (CHEMBL1054000) |
|---|
| IC50 | 5061±n/a nM |
|---|
| Citation |  Lakshminarayana, N; Rajendra Prasad, Y; Gharat, L; Thomas, A; Ravikumar, P; Narayanan, S; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel isochroman carboxylic acid derivatives as potential anti-diabetic agents. Eur J Med Chem44:3147-57 (2009) [PubMed] Article Lakshminarayana, N; Rajendra Prasad, Y; Gharat, L; Thomas, A; Ravikumar, P; Narayanan, S; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel isochroman carboxylic acid derivatives as potential anti-diabetic agents. Eur J Med Chem44:3147-57 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein phosphatase F |
|---|
| Name: | Receptor-type tyrosine-protein phosphatase F |
|---|
| Synonyms: | LAR | Leukocyte common antigen related | Leukocyte common antigen related (LAR) | PTPRF | PTPRF_HUMAN | Receptor-type tyrosine-protein phosphatase F | Receptor-type tyrosine-protein phosphatase F (LAR) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 212869.85 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10586 |
|---|
| Residue: | 1907 |
|---|
| Sequence: | MAPEPAPGRTMVPLVPALVMLGLVAGAHGDSKPVFIKVPEDQTGLSGGVASFVCQATGEP
KPRITWMKKGKKVSSQRFEVIEFDDGAGSVLRIQPLRVQRDEAIYECTATNSLGEINTSA
KLSVLEEEQLPPGFPSIDMGPQLKVVEKARTATMLCAAGGNPDPEISWFKDFLPVDPATS
NGRIKQLRSGALQIESSEESDQGKYECVATNSAGTRYSAPANLYVRVRRVAPRFSIPPSS
QEVMPGGSVNLTCVAVGAPMPYVKWMMGAEELTKEDEMPVGRNVLELSNVVRSANYTCVA
ISSLGMIEATAQVTVKALPKPPIDLVVTETTATSVTLTWDSGNSEPVTYYGIQYRAAGTE
GPFQEVDGVATTRYSIGGLSPFSEYAFRVLAVNSIGRGPPSEAVRARTGEQAPSSPPRRV
QARMLSASTMLVQWEPPEEPNGLVRGYRVYYTPDSRRPPNAWHKHNTDAGLLTTVGSLLP
GITYSLRVLAFTAVGDGPPSPTIQVKTQQGVPAQPADFQAEVESDTRIQLSWLLPPQERI
IMYELVYWAAEDEDQQHKVTFDPTSSYTLEDLKPDTLYRFQLAARSDMGVGVFTPTIEAR
TAQSTPSAPPQKVMCVSMGSTTVRVSWVPPPADSRNGVITQYSVAYEAVDGEDRGRHVVD
GISREHSSWDLVGLEKWTEYRVWVRAHTDVGPGPESSPVLVRTDEDVPSGPPRKVEVEPL
NSTAVHVYWKLPVPSKQHGQIRGYQVTYVRLENGEPRGLPIIQDVMLAEAQWRPEESEDY
ETTISGLTPETTYSVTVAAYTTKGDGARSKPKIVTTTGAVPGRPTMMISTTAMNTALLQW
HPPKELPGELLGYRLQYCRADEARPNTIDFGKDDQHFTVTGLHKGTTYIFRLAAKNRAGL
GEEFEKEIRTPEDLPSGFPQNLHVTGLTTSTTELAWDPPVLAERNGRIISYTVVFRDINS
QQELQNITTDTRFTLTGLKPDTTYDIKVRAWTSKGSGPLSPSIQSRTMPVEQVFAKNFRV
AAAMKTSVLLSWEVPDSYKSAVPFKILYNGQSVEVDGHSMRKLIADLQPNTEYSFVLMNR
GSSAGGLQHLVSIRTAPDLLPHKPLPASAYIEDGRFDLSMPHVQDPSLVRWFYIVVVPID
RVGGSMLTPRWSTPEELELDELLEAIEQGGEEQRRRRRQAERLKPYVAAQLDVLPETFTL
GDKKNYRGFYNRPLSPDLSYQCFVLASLKEPMDQKRYASSPYSDEIVVQVTPAQQQEEPE
MLWVTGPVLAVILIILIVIAILLFKRKRTHSPSSKDEQSIGLKDSLLAHSSDPVEMRRLN
YQTPGMRDHPPIPITDLADNIERLKANDGLKFSQEYESIDPGQQFTWENSNLEVNKPKNR
YANVIAYDHSRVILTSIDGVPGSDYINANYIDGYRKQNAYIATQGPLPETMGDFWRMVWE
QRTATVVMMTRLEEKSRVKCDQYWPARGTETCGLIQVTLLDTVELATYTVRTFALHKSGS
SEKRELRQFQFMAWPDHGVPEYPTPILAFLRRVKACNPLDAGPMVVHCSAGVGRTGCFIV
IDAMLERMKHEKTVDIYGHVTCMRSQRNYMVQTEDQYVFIHEALLEAATCGHTEVPARNL
YAHIQKLGQVPPGESVTAMELEFKLLASSKAHTSRFISANLPCNKFKNRLVNIMPYELTR
VCLQPIRGVEGSDYINASFLDGYRQQKAYIATQGPLAESTEDFWRMLWEHNSTIIVMLTK
LREMGREKCHQYWPAERSARYQYFVVDPMAEYNMPQYILREFKVTDARDGQSRTIRQFQF
TDWPEQGVPKTGEGFIDFIGQVHKTKEQFGQDGPITVHCSAGVGRTGVFITLSIVLERMR
YEGVVDMFQTVKTLRTQRPAMVQTEDQYQLCYRAALEYLGSFDHYAT
|
|
|
|---|
| BDBM50296378 |
|---|
| n/a |
|---|
| Name | BDBM50296378 |
|---|
| Synonyms: | (3S)-7-[2-(2,2-Diphenyl-1,3-dithiolan-4-yl)ethylcarboxamido]-isochroman-3-carboxylic acid | CHEMBL556913 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H27NO4S2 |
|---|
| Mol. Mass. | 505.648 |
|---|
| SMILES | OC(=O)[C@@H]1Cc2ccc(NC(=O)CCC3CSC(S3)(c3ccccc3)c3ccccc3)cc2CO1 |r| |
|---|
| Structure | 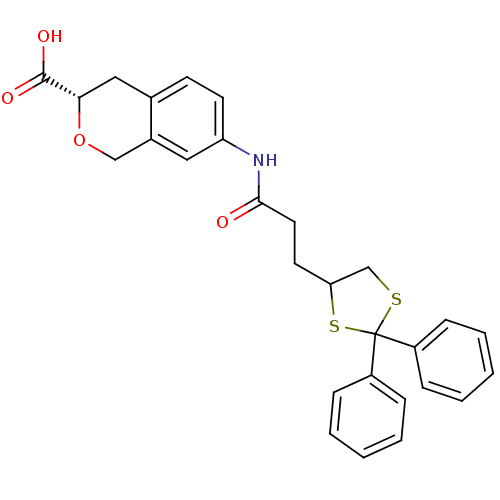
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lakshminarayana, N; Rajendra Prasad, Y; Gharat, L; Thomas, A; Ravikumar, P; Narayanan, S; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel isochroman carboxylic acid derivatives as potential anti-diabetic agents. Eur J Med Chem44:3147-57 (2009) [PubMed] Article
Lakshminarayana, N; Rajendra Prasad, Y; Gharat, L; Thomas, A; Ravikumar, P; Narayanan, S; Srinivasan, CV; Gopalan, B Synthesis and evaluation of some novel isochroman carboxylic acid derivatives as potential anti-diabetic agents. Eur J Med Chem44:3147-57 (2009) [PubMed] Article