| Reaction Details |
|---|
 | Report a problem with these data |
| Target | DNA topoisomerase 2-alpha |
|---|
| Ligand | BDBM50123623 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_588198 (CHEMBL1050089) |
|---|
| IC50 | 60±n/a nM |
|---|
| Citation |  Azéma, J; Guidetti, B; Dewelle, J; Le Calve, B; Mijatovic, T; Korolyov, A; Vaysse, J; Malet-Martino, M; Martino, R; Kiss, R 7-((4-Substituted)piperazin-1-yl) derivatives of ciprofloxacin: synthesis and in vitro biological evaluation as potential antitumor agents. Bioorg Med Chem17:5396-407 (2009) [PubMed] Article Azéma, J; Guidetti, B; Dewelle, J; Le Calve, B; Mijatovic, T; Korolyov, A; Vaysse, J; Malet-Martino, M; Martino, R; Kiss, R 7-((4-Substituted)piperazin-1-yl) derivatives of ciprofloxacin: synthesis and in vitro biological evaluation as potential antitumor agents. Bioorg Med Chem17:5396-407 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| DNA topoisomerase 2-alpha |
|---|
| Name: | DNA topoisomerase 2-alpha |
|---|
| Synonyms: | DNA topoisomerase 2-alpha | DNA topoisomerase II | DNA topoisomerase II (Topo II) | DNA topoisomerase II alpha | DNA topoisomerase II, alpha isozyme | TOP2 | TOP2A | TOP2A_HUMAN | Topoisomerase I/II | Topoisomerase II alpha (HuTopoIIα) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 174415.30 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11388 |
|---|
| Residue: | 1531 |
|---|
| Sequence: | MEVSPLQPVNENMQVNKIKKNEDAKKRLSVERIYQKKTQLEHILLRPDTYIGSVELVTQQ
MWVYDEDVGINYREVTFVPGLYKIFDEILVNAADNKQRDPKMSCIRVTIDPENNLISIWN
NGKGIPVVEHKVEKMYVPALIFGQLLTSSNYDDDEKKVTGGRNGYGAKLCNIFSTKFTVE
TASREYKKMFKQTWMDNMGRAGEMELKPFNGEDYTCITFQPDLSKFKMQSLDKDIVALMV
RRAYDIAGSTKDVKVFLNGNKLPVKGFRSYVDMYLKDKLDETGNSLKVIHEQVNHRWEVC
LTMSEKGFQQISFVNSIATSKGGRHVDYVADQIVTKLVDVVKKKNKGGVAVKAHQVKNHM
WIFVNALIENPTFDSQTKENMTLQPKSFGSTCQLSEKFIKAAIGCGIVESILNWVKFKAQ
VQLNKKCSAVKHNRIKGIPKLDDANDAGGRNSTECTLILTEGDSAKTLAVSGLGVVGRDK
YGVFPLRGKILNVREASHKQIMENAEINNIIKIVGLQYKKNYEDEDSLKTLRYGKIMIMT
DQDQDGSHIKGLLINFIHHNWPSLLRHRFLEEFITPIVKVSKNKQEMAFYSLPEFEEWKS
STPNHKKWKVKYYKGLGTSTSKEAKEYFADMKRHRIQFKYSGPEDDAAISLAFSKKQIDD
RKEWLTNFMEDRRQRKLLGLPEDYLYGQTTTYLTYNDFINKELILFSNSDNERSIPSMVD
GLKPGQRKVLFTCFKRNDKREVKVAQLAGSVAEMSSYHHGEMSLMMTIINLAQNFVGSNN
LNLLQPIGQFGTRLHGGKDSASPRYIFTMLSSLARLLFPPKDDHTLKFLYDDNQRVEPEW
YIPIIPMVLINGAEGIGTGWSCKIPNFDVREIVNNIRRLMDGEEPLPMLPSYKNFKGTIE
ELAPNQYVISGEVAILNSTTIEISELPVRTWTQTYKEQVLEPMLNGTEKTPPLITDYREY
HTDTTVKFVVKMTEEKLAEAERVGLHKVFKLQTSLTCNSMVLFDHVGCLKKYDTVLDILR
DFFELRLKYYGLRKEWLLGMLGAESAKLNNQARFILEKIDGKIIIENKPKKELIKVLIQR
GYDSDPVKAWKEAQQKVPDEEENEESDNEKETEKSDSVTDSGPTFNYLLDMPLWYLTKEK
KDELCRLRNEKEQELDTLKRKSPSDLWKEDLATFIEELEAVEAKEKQDEQVGLPGKGGKA
KGKKTQMAEVLPSPRGQRVIPRITIEMKAEAEKKNKKKIKNENTEGSPQEDGVELEGLKQ
RLEKKQKREPGTKTKKQTTLAFKPIKKGKKRNPWSDSESDRSSDESNFDVPPRETEPRRA
ATKTKFTMDLDSDEDFSDFDEKTDDEDFVPSDASPPKTKTSPKLSNKELKPQKSVVSDLE
ADDVKGSVPLSSSPPATHFPDETEITNPVPKKNVTVKKTAAKSQSSTSTTGAKKRAAPKG
TKRDPALNSGVSQKPDPAKTKNRRKRKPSTSDDSDSNFEKIVSKAVTSKKSKGESDDFHM
DFDSAVAPRAKSVRAKKPIKYLEESDEDDLF
|
|
|
|---|
| BDBM50123623 |
|---|
| n/a |
|---|
| Name | BDBM50123623 |
|---|
| Synonyms: | (R)-13-(3-aminopyrrolidin-1-yl)-12-fluoro-10-oxo-10H-naphtho[1,2-c]pyrido[3,2,1-kl]phenoxazine-9-carboxylic acid | 13-[3-amino-(3R)-tetrahydro-1H-1-pyrrolyl]-12-fluoro-10-oxo-10H-naphtho[1,2-c]pyrido[3,2,1-kl]phenoxazine-9-carboxylic acid | CHEMBL346068 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H20FN3O4 |
|---|
| Mol. Mass. | 481.4745 |
|---|
| SMILES | N[C@@H]1CCN(C1)c1c(F)cc2c3c1oc1c4ccc5ccccc5c4ccc1n3cc(C(O)=O)c2=O |
|---|
| Structure | 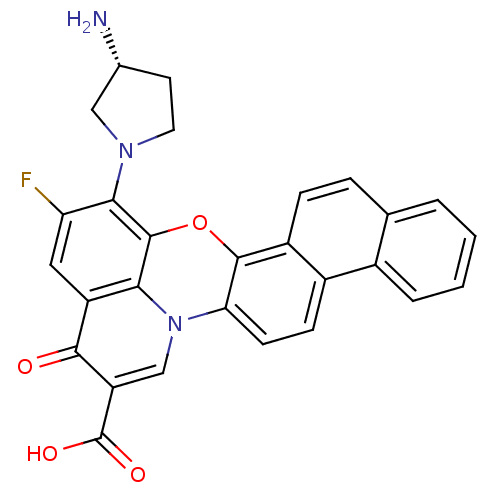
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Azéma, J; Guidetti, B; Dewelle, J; Le Calve, B; Mijatovic, T; Korolyov, A; Vaysse, J; Malet-Martino, M; Martino, R; Kiss, R 7-((4-Substituted)piperazin-1-yl) derivatives of ciprofloxacin: synthesis and in vitro biological evaluation as potential antitumor agents. Bioorg Med Chem17:5396-407 (2009) [PubMed] Article
Azéma, J; Guidetti, B; Dewelle, J; Le Calve, B; Mijatovic, T; Korolyov, A; Vaysse, J; Malet-Martino, M; Martino, R; Kiss, R 7-((4-Substituted)piperazin-1-yl) derivatives of ciprofloxacin: synthesis and in vitro biological evaluation as potential antitumor agents. Bioorg Med Chem17:5396-407 (2009) [PubMed] Article