| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen synthase kinase-3 beta |
|---|
| Ligand | BDBM50299093 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_591611 (CHEMBL1042364) |
|---|
| IC50 | 110±n/a nM |
|---|
| Citation |  Saitoh, M; Kunitomo, J; Kimura, E; Iwashita, H; Uno, Y; Onishi, T; Uchiyama, N; Kawamoto, T; Tanaka, T; Mol, CD; Dougan, DR; Textor, GP; Snell, GP; Takizawa, M; Itoh, F; Kori, M 2-{3-[4-(Alkylsulfinyl)phenyl]-1-benzofuran-5-yl}-5-methyl-1,3,4-oxadiazole derivatives as novel inhibitors of glycogen synthase kinase-3beta with good brain permeability. J Med Chem52:6270-86 (2009) [PubMed] Article Saitoh, M; Kunitomo, J; Kimura, E; Iwashita, H; Uno, Y; Onishi, T; Uchiyama, N; Kawamoto, T; Tanaka, T; Mol, CD; Dougan, DR; Textor, GP; Snell, GP; Takizawa, M; Itoh, F; Kori, M 2-{3-[4-(Alkylsulfinyl)phenyl]-1-benzofuran-5-yl}-5-methyl-1,3,4-oxadiazole derivatives as novel inhibitors of glycogen synthase kinase-3beta with good brain permeability. J Med Chem52:6270-86 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen synthase kinase-3 beta |
|---|
| Name: | Glycogen synthase kinase-3 beta |
|---|
| Synonyms: | GSK-3 beta | GSK-3, beta | GSK3B | GSK3B_HUMAN | Glycogen synthase kinase 3 beta (GSK3B) | Glycogen synthase kinase 3-beta (GSK3B) | Glycogen synthase kinase-3 beta (GSK-3B) | Glycogen synthase kinase-3 beta (GSK3 Beta) | Glycogen synthase kinase-3 beta (GSK3B) | Glycogen synthase kinase-3B (GSK-3B) | Glycogen synthase kinase-3beta (GSK3B) | Serine/threonine-protein kinase GSK3B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 46756.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49841 |
|---|
| Residue: | 420 |
|---|
| Sequence: | MSGRPRTTSFAESCKPVQQPSAFGSMKVSRDKDGSKVTTVVATPGQGPDRPQEVSYTDTK
VIGNGSFGVVYQAKLCDSGELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFYSSG
EKKDEVYLNLVLDYVPETVYRVARHYSRAKQTLPVIYVKLYMYQLFRSLAYIHSFGICHR
DIKPQNLLLDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSSIDV
WSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIKAHP
WTKVFRPRTPPEAIALCSRLLEYTPTARLTPLEACAHSFFDELRDPNVKLPNGRDTPALF
NFTTQELSSNPPLATILIPPHARIQAAASTPTNATAASDANTGDRGQTNNAASASASNST
|
|
|
|---|
| BDBM50299093 |
|---|
| n/a |
|---|
| Name | BDBM50299093 |
|---|
| Synonyms: | 2-[3-(4-Fluorophenyl)-1-benzofuran-5-yl]-5-methyl-1,3,4-oxadiazol | CHEMBL573007 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H13FO2 |
|---|
| Mol. Mass. | 292.3037 |
|---|
| SMILES | Cc1ccc(o1)-c1ccc2occ(-c3ccc(F)cc3)c2c1 |
|---|
| Structure | 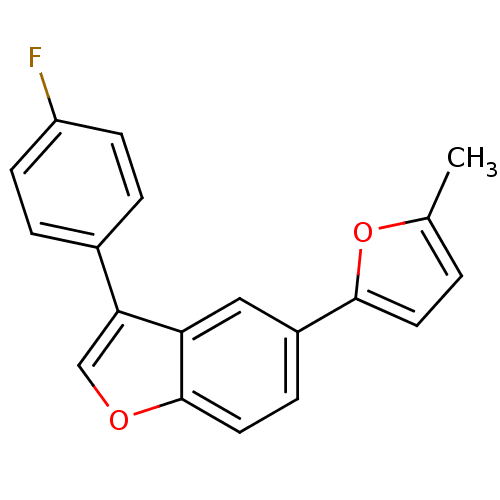
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Saitoh, M; Kunitomo, J; Kimura, E; Iwashita, H; Uno, Y; Onishi, T; Uchiyama, N; Kawamoto, T; Tanaka, T; Mol, CD; Dougan, DR; Textor, GP; Snell, GP; Takizawa, M; Itoh, F; Kori, M 2-{3-[4-(Alkylsulfinyl)phenyl]-1-benzofuran-5-yl}-5-methyl-1,3,4-oxadiazole derivatives as novel inhibitors of glycogen synthase kinase-3beta with good brain permeability. J Med Chem52:6270-86 (2009) [PubMed] Article
Saitoh, M; Kunitomo, J; Kimura, E; Iwashita, H; Uno, Y; Onishi, T; Uchiyama, N; Kawamoto, T; Tanaka, T; Mol, CD; Dougan, DR; Textor, GP; Snell, GP; Takizawa, M; Itoh, F; Kori, M 2-{3-[4-(Alkylsulfinyl)phenyl]-1-benzofuran-5-yl}-5-methyl-1,3,4-oxadiazole derivatives as novel inhibitors of glycogen synthase kinase-3beta with good brain permeability. J Med Chem52:6270-86 (2009) [PubMed] Article