| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone-lysine N-methyltransferase EHMT1 |
|---|
| Ligand | BDBM50300041 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_593238 (CHEMBL1047554) |
|---|
| IC50 | 20±n/a nM |
|---|
| Citation |  Liu, F; Chen, X; Allali-Hassani, A; Quinn, AM; Wasney, GA; Dong, A; Barsyte, D; Kozieradzki, I; Senisterra, G; Chau, I; Siarheyeva, A; Kireev, DB; Jadhav, A; Herold, JM; Frye, SV; Arrowsmith, CH; Brown, PJ; Simeonov, A; Vedadi, M; Jin, J Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a. J Med Chem52:7950-3 (2009) [PubMed] Article Liu, F; Chen, X; Allali-Hassani, A; Quinn, AM; Wasney, GA; Dong, A; Barsyte, D; Kozieradzki, I; Senisterra, G; Chau, I; Siarheyeva, A; Kireev, DB; Jadhav, A; Herold, JM; Frye, SV; Arrowsmith, CH; Brown, PJ; Simeonov, A; Vedadi, M; Jin, J Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a. J Med Chem52:7950-3 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone-lysine N-methyltransferase EHMT1 |
|---|
| Name: | Histone-lysine N-methyltransferase EHMT1 |
|---|
| Synonyms: | EHMT1 | EHMT1_HUMAN | EUHMTASE1 | Eu-HMTase1 | Euchromatic histone-lysine N-methyltransferase 1 | G9a-like protein 1 | GLP | GLP1 | H3-K9-HMTase 5 | Histone H3-K9 methyltransferase 5 | Histone-lysine N-methyltransferase EHMT1/EHMT2 | KIAA1876 | KMT1D | Lysine N-methyltransferase 1D |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 141443.67 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1450367 |
|---|
| Residue: | 1298 |
|---|
| Sequence: | MAAADAEAVPARGEPQQDCCVKTELLGEETPMAADEGSAEKQAGEAHMAADGETNGSCEN
SDASSHANAAKHTQDSARVNPQDGTNTLTRIAENGVSERDSEAAKQNHVTADDFVQTSVI
GSNGYILNKPALQAQPLRTTSTLASSLPGHAAKTLPGGAGKGRTPSAFPQTPAAPPATLG
EGSADTEDRKLPAPGADVKVHRARKTMPKSVVGLHAASKDPREVREARDHKEPKEEINKN
ISDFGRQQLLPPFPSLHQSLPQNQCYMATTKSQTACLPFVLAAAVSRKKKRRMGTYSLVP
KKKTKVLKQRTVIEMFKSITHSTVGSKGEKDLGASSLHVNGESLEMDSDEDDSEELEEDD
GHGAEQAAAFPTEDSRTSKESMSEADRAQKMDGESEEEQESVDTGEEEEGGDESDLSSES
SIKKKFLKRKGKTDSPWIKPARKRRRRSRKKPSGALGSESYKSSAGSAEQTAPGDSTGYM
EVSLDSLDLRVKGILSSQAEGLANGPDVLETDGLQEVPLCSCRMETPKSREITTLANNQC
MATESVDHELGRCTNSVVKYELMRPSNKAPLLVLCEDHRGRMVKHQCCPGCGYFCTAGNF
MECQPESSISHRFHKDCASRVNNASYCPHCGEESSKAKEVTIAKADTTSTVTPVPGQEKG
SALEGRADTTTGSAAGPPLSEDDKLQGAASHVPEGFDPTGPAGLGRPTPGLSQGPGKETL
ESALIALDSEKPKKLRFHPKQLYFSARQGELQKVLLMLVDGIDPNFKMEHQNKRSPLHAA
AEAGHVDICHMLVQAGANIDTCSEDQRTPLMEAAENNHLEAVKYLIKAGALVDPKDAEGS
TCLHLAAKKGHYEVVQYLLSNGQMDVNCQDDGGWTPMIWATEYKHVDLVKLLLSKGSDIN
IRDNEENICLHWAAFSGCVDIAEILLAAKCDLHAVNIHGDSPLHIAARENRYDCVVLFLS
RDSDVTLKNKEGETPLQCASLNSQVWSALQMSKALQDSAPDRPSPVERIVSRDIARGYER
IPIPCVNAVDSEPCPSNYKYVSQNCVTSPMNIDRNITHLQYCVCIDDCSSSNCMCGQLSM
RCWYDKDGRLLPEFNMAEPPLIFECNHACSCWRNCRNRVVQNGLRARLQLYRTRDMGWGV
RSLQDIPPGTFVCEYVGELISDSEADVREEDSYLFDLDNKDGEVYCIDARFYGNVSRFIN
HHCEPNLVPVRVFMAHQDLRFPRIAFFSTRLIEAGEQLGFDYGERFWDIKGKLFSCRCGS
PKCRHSSAALAQRQASAAQEAQEDGLPDTSSAAAADPL
|
|
|
|---|
| BDBM50300041 |
|---|
| n/a |
|---|
| Name | BDBM50300041 |
|---|
| Synonyms: | 7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl-1,4-diazepan-1-yl)-N-(1-methylpiperidin-4-yl)quinazolin-4-amine | 7-[3-(dimethylamino)propoxy]-6-methoxy-2-(4-methyl-1,4-diazepan-1-yl)-N-(1-methylpiperidin-4-yl)quinazolin-4-amine | CHEMBL576781 | UNC0224 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H43N7O2 |
|---|
| Mol. Mass. | 485.6653 |
|---|
| SMILES | COc1cc2c(NC3CCN(C)CC3)nc(nc2cc1OCCCN(C)C)N1CCCN(C)CC1 |
|---|
| Structure | 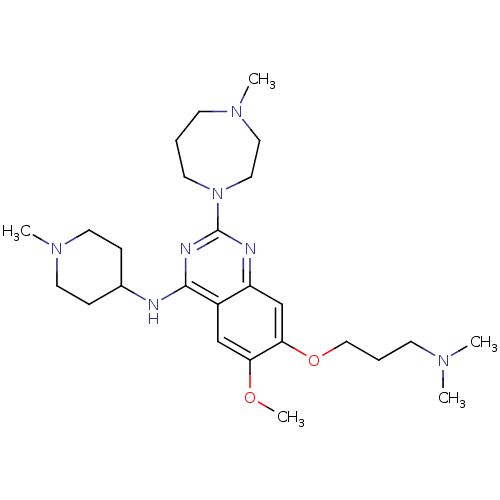
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, F; Chen, X; Allali-Hassani, A; Quinn, AM; Wasney, GA; Dong, A; Barsyte, D; Kozieradzki, I; Senisterra, G; Chau, I; Siarheyeva, A; Kireev, DB; Jadhav, A; Herold, JM; Frye, SV; Arrowsmith, CH; Brown, PJ; Simeonov, A; Vedadi, M; Jin, J Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a. J Med Chem52:7950-3 (2009) [PubMed] Article
Liu, F; Chen, X; Allali-Hassani, A; Quinn, AM; Wasney, GA; Dong, A; Barsyte, D; Kozieradzki, I; Senisterra, G; Chau, I; Siarheyeva, A; Kireev, DB; Jadhav, A; Herold, JM; Frye, SV; Arrowsmith, CH; Brown, PJ; Simeonov, A; Vedadi, M; Jin, J Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a. J Med Chem52:7950-3 (2009) [PubMed] Article