| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50301735 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_598026 (CHEMBL1046295) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Dow, RL; Hadcock, JR; Scott, DO; Schneider, SR; Paight, ES; Iredale, PA; Carpino, PA; Griffith, DA; Hammond, M; Dasilva-Jardine, P Bioisosteric replacement of the hydrazide pharmacophore of the cannabinoid-1 receptor antagonist SR141716A. Part I: potent, orally-active 1,4-disubstituted imidazoles. Bioorg Med Chem Lett19:5351-4 (2009) [PubMed] Article Dow, RL; Hadcock, JR; Scott, DO; Schneider, SR; Paight, ES; Iredale, PA; Carpino, PA; Griffith, DA; Hammond, M; Dasilva-Jardine, P Bioisosteric replacement of the hydrazide pharmacophore of the cannabinoid-1 receptor antagonist SR141716A. Part I: potent, orally-active 1,4-disubstituted imidazoles. Bioorg Med Chem Lett19:5351-4 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50301735 |
|---|
| n/a |
|---|
| Name | BDBM50301735 |
|---|
| Synonyms: | 1-(2-chlorophenyl)-5-(4-chlorophenyl)-3-(1-isopropyl-1H-imidazol-4-yl)-4-methyl-1H-pyrazole | CHEMBL584261 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H20Cl2N4 |
|---|
| Mol. Mass. | 411.327 |
|---|
| SMILES | CC(C)n1cnc(c1)-c1nn(c(c1C)-c1ccc(Cl)cc1)-c1ccccc1Cl |
|---|
| Structure | 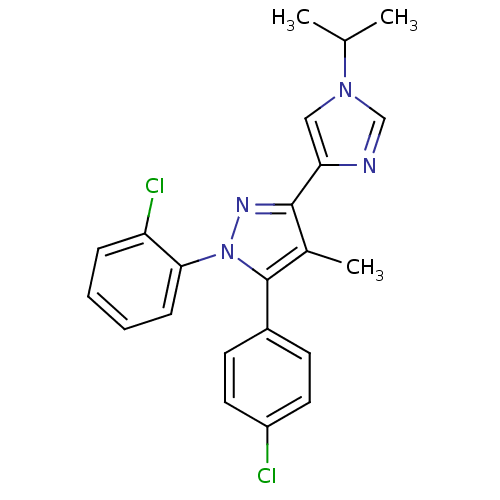
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Dow, RL; Hadcock, JR; Scott, DO; Schneider, SR; Paight, ES; Iredale, PA; Carpino, PA; Griffith, DA; Hammond, M; Dasilva-Jardine, P Bioisosteric replacement of the hydrazide pharmacophore of the cannabinoid-1 receptor antagonist SR141716A. Part I: potent, orally-active 1,4-disubstituted imidazoles. Bioorg Med Chem Lett19:5351-4 (2009) [PubMed] Article
Dow, RL; Hadcock, JR; Scott, DO; Schneider, SR; Paight, ES; Iredale, PA; Carpino, PA; Griffith, DA; Hammond, M; Dasilva-Jardine, P Bioisosteric replacement of the hydrazide pharmacophore of the cannabinoid-1 receptor antagonist SR141716A. Part I: potent, orally-active 1,4-disubstituted imidazoles. Bioorg Med Chem Lett19:5351-4 (2009) [PubMed] Article