| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urotensin-2 receptor |
|---|
| Ligand | BDBM50302270 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_596665 (CHEMBL1048827) |
|---|
| IC50 | 1±n/a nM |
|---|
| Citation |  Lawson, EC; Luci, DK; Ghosh, S; Kinney, WA; Reynolds, CH; Qi, J; Smith, CE; Wang, Y; Minor, LK; Haertlein, BJ; Parry, TJ; Damiano, BP; Maryanoff, BE Nonpeptide urotensin-II receptor antagonists: a new ligand class based on piperazino-phthalimide and piperazino-isoindolinone subunits. J Med Chem52:7432-45 (2009) [PubMed] Article Lawson, EC; Luci, DK; Ghosh, S; Kinney, WA; Reynolds, CH; Qi, J; Smith, CE; Wang, Y; Minor, LK; Haertlein, BJ; Parry, TJ; Damiano, BP; Maryanoff, BE Nonpeptide urotensin-II receptor antagonists: a new ligand class based on piperazino-phthalimide and piperazino-isoindolinone subunits. J Med Chem52:7432-45 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Urotensin-2 receptor |
|---|
| Name: | Urotensin-2 receptor |
|---|
| Synonyms: | G-protein coupled receptor 14 | G-protein coupled sensory epithelial neuropeptide-like receptor | Gpr14 | Senr | UR-II-R | UR2R_RAT | Urotensin-II | Uts2r |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 42725.34 |
|---|
| Organism: | RAT |
|---|
| Description: | Urotensin-II UTS2R RAT::P49684 |
|---|
| Residue: | 386 |
|---|
| Sequence: | MALSLESTTSFHMLTVSGSTVTELPGDSNVSLNSSWSGPTDPSSLKDLVATGVIGAVLSA
MGVVGMVGNVYTLVVMCRFLRASASMYVYVVNLALADLLYLLSIPFIIATYVTKDWHFGD
VGCRVLFSLDFLTMHASIFTLTIMSSERYAAVLRPLDTVQRSKGYRKLLVLGTWLLALLL
TLPMMLAIQLVRRGSKSLCLPAWGPRAHRTYLTLLFGTSIVGPGLVIGLLYVRLARAYWL
SQQASFKQTRRLPNPRVLYLILGIVLLFWACFLPFWLWQLLAQYHEAMPLTPETARIVNY
LTTCLTYGNSCINPFLYTLLTKNYREYLRGRQRSLGSSCHSPGSPGSFLPSRVHLQQDSG
RSLSSSSQQATETLMLSPVPRNGALL
|
|
|
|---|
| BDBM50302270 |
|---|
| n/a |
|---|
| Name | BDBM50302270 |
|---|
| Synonyms: | (R)-2-(4-(6-chloropyrazin-2-ylamino)-1-(3,4-dimethoxyphenyl)butyl)-4-(4-ethylpiperazin-1-yl)isoindolin-1-one | CHEMBL566056 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H37ClN6O3 |
|---|
| Mol. Mass. | 565.106 |
|---|
| SMILES | CCN1CCN(CC1)c1cccc2C(=O)N(Cc12)[C@H](CCCNc1cncc(Cl)n1)c1ccc(OC)c(OC)c1 |r| |
|---|
| Structure | 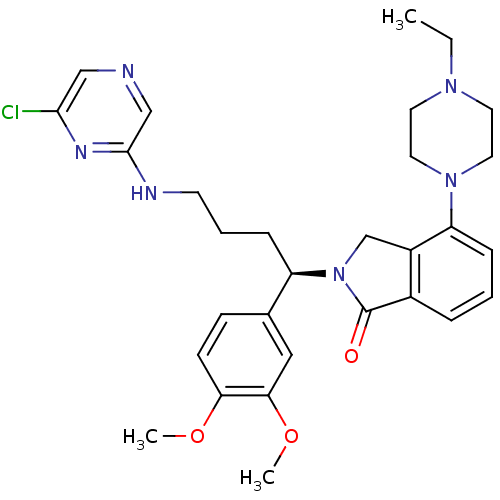
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lawson, EC; Luci, DK; Ghosh, S; Kinney, WA; Reynolds, CH; Qi, J; Smith, CE; Wang, Y; Minor, LK; Haertlein, BJ; Parry, TJ; Damiano, BP; Maryanoff, BE Nonpeptide urotensin-II receptor antagonists: a new ligand class based on piperazino-phthalimide and piperazino-isoindolinone subunits. J Med Chem52:7432-45 (2009) [PubMed] Article
Lawson, EC; Luci, DK; Ghosh, S; Kinney, WA; Reynolds, CH; Qi, J; Smith, CE; Wang, Y; Minor, LK; Haertlein, BJ; Parry, TJ; Damiano, BP; Maryanoff, BE Nonpeptide urotensin-II receptor antagonists: a new ligand class based on piperazino-phthalimide and piperazino-isoindolinone subunits. J Med Chem52:7432-45 (2009) [PubMed] Article