| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Amine oxidase [flavin-containing] B |
|---|
| Ligand | BDBM50304158 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_601026 (CHEMBL1069163) |
|---|
| IC50 | 2610±n/a nM |
|---|
| Citation |  Gökhan-Kelekçi, N; Simsek, OO; Ercan, A; Yelekçi, K; Sahin, ZS; Isik, S; Uçar, G; Bilgin, AA Synthesis and molecular modeling of some novel hexahydroindazole derivatives as potent monoamine oxidase inhibitors. Bioorg Med Chem17:6761-72 (2009) [PubMed] Article Gökhan-Kelekçi, N; Simsek, OO; Ercan, A; Yelekçi, K; Sahin, ZS; Isik, S; Uçar, G; Bilgin, AA Synthesis and molecular modeling of some novel hexahydroindazole derivatives as potent monoamine oxidase inhibitors. Bioorg Med Chem17:6761-72 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Amine oxidase [flavin-containing] B |
|---|
| Name: | Amine oxidase [flavin-containing] B |
|---|
| Synonyms: | AOFB_RAT | Amine oxidase (flavin-containing) B | Amine oxidase [flavin-containing] B | Maob | Monoamine Oxidase Type B (MAO-B) | Monoamine oxidase | Monoamine oxidase B (MAO-B) | Monoamine oxidase B (rMAO-B) | Monoamine oxidase type B (MAOB) | Monoamine oxidase-B (MAO-B) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58469.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | P19643 |
|---|
| Residue: | 520 |
|---|
| Sequence: | MSNKCDVIVVGGGISGMAAAKLLHDCGLSVVVLEARDRVGGRTYTIRNKNVKYVDLGGSY
VGPTQNRILRLAKELGLETYKVNEVERLIHFVKGKSYAFRGPFPPVWNPITYLDYNNLWR
TMDEMGQEIPSDAPWKAPLAEEWDYMTMKELLDKICWTNSTKQIATLFVNLCVTAETHEV
SALWFLWYVKQCGGTTRIISTTNGGQERKFIGGSGQVSERIKDILGDRVKLERPVIHIDQ
TGENVVVKTLNHEIYEAKYVISAIPPVLGMKIHHSPPLPILRNQLITRVPLGSVIKCMVY
YKEPFWRKKDFCGTMVIEGEEAPIAYTLDDTKPDGSCAAIMGFILAHKARKLVRLTKEER
LRKLCELYAKVLNSQEALQPVHYEEKNWCEEQYSGGCYTAYFPPGILTQYGRVLRQPVGK
IFFAGTETASHWSGYMEGAVEAGERAAREILHAIGKIPEDEIWQPEPESVDVPARPITNT
FLERHLPSVPGLLKLLGLTTILSATALGFLAHKKGLFVRF
|
|
|
|---|
| BDBM50304158 |
|---|
| n/a |
|---|
| Name | BDBM50304158 |
|---|
| Synonyms: | 3-(2-Thienyl)-2-(N-methylthiocarbamoyl)-3,3a,4,5,6,7-hexahydro-2H-indazol | CHEMBL596339 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H17N3S2 |
|---|
| Mol. Mass. | 279.424 |
|---|
| SMILES | CNC(=S)N1N=C2CCCCC2C1c1cccs1 |t:5| |
|---|
| Structure | 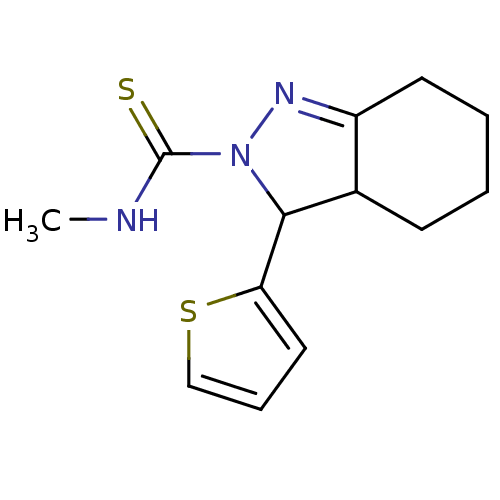
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gökhan-Kelekçi, N; Simsek, OO; Ercan, A; Yelekçi, K; Sahin, ZS; Isik, S; Uçar, G; Bilgin, AA Synthesis and molecular modeling of some novel hexahydroindazole derivatives as potent monoamine oxidase inhibitors. Bioorg Med Chem17:6761-72 (2009) [PubMed] Article
Gökhan-Kelekçi, N; Simsek, OO; Ercan, A; Yelekçi, K; Sahin, ZS; Isik, S; Uçar, G; Bilgin, AA Synthesis and molecular modeling of some novel hexahydroindazole derivatives as potent monoamine oxidase inhibitors. Bioorg Med Chem17:6761-72 (2009) [PubMed] Article