| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen synthase kinase-3 beta |
|---|
| Ligand | BDBM50310459 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_619400 (CHEMBL1104158) |
|---|
| Ki | 290±n/a nM |
|---|
| Citation |  Bandarage, U; Hare, B; Parsons, J; Pham, L; Marhefka, C; Bemis, G; Tang, Q; Moody, CS; Rodems, S; Shah, S; Adams, C; Bravo, J; Charonnet, E; Savic, V; Come, JH; Green, J 4-(Benzimidazol-2-yl)-1,2,5-oxadiazol-3-ylamine derivatives: potent and selective p70S6 kinase inhibitors. Bioorg Med Chem Lett19:5191-4 (2009) [PubMed] Article Bandarage, U; Hare, B; Parsons, J; Pham, L; Marhefka, C; Bemis, G; Tang, Q; Moody, CS; Rodems, S; Shah, S; Adams, C; Bravo, J; Charonnet, E; Savic, V; Come, JH; Green, J 4-(Benzimidazol-2-yl)-1,2,5-oxadiazol-3-ylamine derivatives: potent and selective p70S6 kinase inhibitors. Bioorg Med Chem Lett19:5191-4 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen synthase kinase-3 beta |
|---|
| Name: | Glycogen synthase kinase-3 beta |
|---|
| Synonyms: | GSK-3 beta | GSK-3, beta | GSK3B | GSK3B_HUMAN | Glycogen synthase kinase 3 beta (GSK3B) | Glycogen synthase kinase 3-beta (GSK3B) | Glycogen synthase kinase-3 beta (GSK-3B) | Glycogen synthase kinase-3 beta (GSK3 Beta) | Glycogen synthase kinase-3 beta (GSK3B) | Glycogen synthase kinase-3B (GSK-3B) | Glycogen synthase kinase-3beta (GSK3B) | Serine/threonine-protein kinase GSK3B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 46756.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49841 |
|---|
| Residue: | 420 |
|---|
| Sequence: | MSGRPRTTSFAESCKPVQQPSAFGSMKVSRDKDGSKVTTVVATPGQGPDRPQEVSYTDTK
VIGNGSFGVVYQAKLCDSGELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFYSSG
EKKDEVYLNLVLDYVPETVYRVARHYSRAKQTLPVIYVKLYMYQLFRSLAYIHSFGICHR
DIKPQNLLLDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSSIDV
WSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIKAHP
WTKVFRPRTPPEAIALCSRLLEYTPTARLTPLEACAHSFFDELRDPNVKLPNGRDTPALF
NFTTQELSSNPPLATILIPPHARIQAAASTPTNATAASDANTGDRGQTNNAASASASNST
|
|
|
|---|
| BDBM50310459 |
|---|
| n/a |
|---|
| Name | BDBM50310459 |
|---|
| Synonyms: | 4-(1-(cyclopropylmethyl)-1H-benzo[d]imidazol-2-yl)-1,2,5-oxadiazol-3-amine | CHEMBL1078930 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H13N5O |
|---|
| Mol. Mass. | 255.2752 |
|---|
| SMILES | Nc1nonc1-c1nc2ccccc2n1CC1CC1 |
|---|
| Structure | 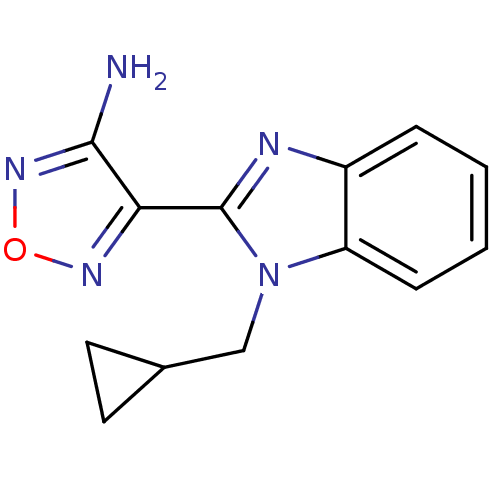
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bandarage, U; Hare, B; Parsons, J; Pham, L; Marhefka, C; Bemis, G; Tang, Q; Moody, CS; Rodems, S; Shah, S; Adams, C; Bravo, J; Charonnet, E; Savic, V; Come, JH; Green, J 4-(Benzimidazol-2-yl)-1,2,5-oxadiazol-3-ylamine derivatives: potent and selective p70S6 kinase inhibitors. Bioorg Med Chem Lett19:5191-4 (2009) [PubMed] Article
Bandarage, U; Hare, B; Parsons, J; Pham, L; Marhefka, C; Bemis, G; Tang, Q; Moody, CS; Rodems, S; Shah, S; Adams, C; Bravo, J; Charonnet, E; Savic, V; Come, JH; Green, J 4-(Benzimidazol-2-yl)-1,2,5-oxadiazol-3-ylamine derivatives: potent and selective p70S6 kinase inhibitors. Bioorg Med Chem Lett19:5191-4 (2009) [PubMed] Article