| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aurora kinase B |
|---|
| Ligand | BDBM50310622 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_620386 (CHEMBL1112281) |
|---|
| IC50 | 21±n/a nM |
|---|
| Citation |  Zhong, M; Bui, M; Shen, W; Baskaran, S; Allen, DA; Elling, RA; Flanagan, WM; Fung, AD; Hanan, EJ; Harris, SO; Heumann, SA; Hoch, U; Ivy, SN; Jacobs, JW; Lam, S; Lee, H; McDowell, RS; Oslob, JD; Purkey, HE; Romanowski, MJ; Silverman, JA; Tangonan, BT; Taverna, P; Yang, W; Yoburn, JC; Yu, CH; Zimmerman, KM; O'Brien, T; Lew, W 2-Aminobenzimidazoles as potent Aurora kinase inhibitors. Bioorg Med Chem Lett19:5158-61 (2009) [PubMed] Article Zhong, M; Bui, M; Shen, W; Baskaran, S; Allen, DA; Elling, RA; Flanagan, WM; Fung, AD; Hanan, EJ; Harris, SO; Heumann, SA; Hoch, U; Ivy, SN; Jacobs, JW; Lam, S; Lee, H; McDowell, RS; Oslob, JD; Purkey, HE; Romanowski, MJ; Silverman, JA; Tangonan, BT; Taverna, P; Yang, W; Yoburn, JC; Yu, CH; Zimmerman, KM; O'Brien, T; Lew, W 2-Aminobenzimidazoles as potent Aurora kinase inhibitors. Bioorg Med Chem Lett19:5158-61 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aurora kinase B |
|---|
| Name: | Aurora kinase B |
|---|
| Synonyms: | AIK2 | AIM-1 | AIM1 | AIRK2 | ARK2 | AURKB | AURKB_HUMAN | Aurora B kinase (aurB) | Aurora B-INCENP | Aurora kinase 2 | Aurora kinase B (AURKB) | Aurora-related kinase 2 | STK-1 | STK1 | STK12 | STK5 | Serine/threonine-protein kinase aurora B |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 39327.72 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q96GD4 |
|---|
| Residue: | 344 |
|---|
| Sequence: | MAQKENSYPWPYGRQTAPSGLSTLPQRVLRKEPVTPSALVLMSRSNVQPTAAPGQKVMEN
SSGTPDILTRHFTIDDFEIGRPLGKGKFGNVYLAREKKSHFIVALKVLFKSQIEKEGVEH
QLRREIEIQAHLHHPNILRLYNYFYDRRRIYLILEYAPRGELYKELQKSCTFDEQRTATI
MEELADALMYCHGKKVIHRDIKPENLLLGLKGELKIADFGWSVHAPSLRRKTMCGTLDYL
PPEMIEGRMHNEKVDLWCIGVLCYELLVGNPPFESASHNETYRRIVKVDLKFPASVPMGA
QDLISKLLRHNPSERLPLAQVSAHPWVRANSRRVLPPSALQSVA
|
|
|
|---|
| BDBM50310622 |
|---|
| n/a |
|---|
| Name | BDBM50310622 |
|---|
| Synonyms: | CHEMBL1078061 | N-(3-fluoro-4-(5-(trifluoromethyl)-1H-benzo[d]imidazol-2-ylamino)phenethyl)thieno[3,2-d]pyrimidin-4-amine |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H16F4N6S |
|---|
| Mol. Mass. | 472.461 |
|---|
| SMILES | Fc1cc(CCNc2ncnc3ccsc23)ccc1Nc1nc2ccc(cc2[nH]1)C(F)(F)F |
|---|
| Structure | 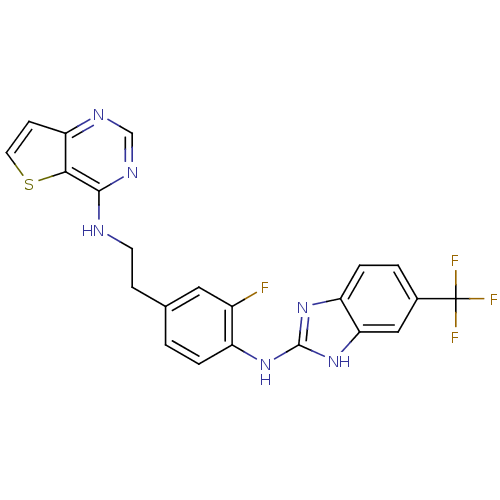
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhong, M; Bui, M; Shen, W; Baskaran, S; Allen, DA; Elling, RA; Flanagan, WM; Fung, AD; Hanan, EJ; Harris, SO; Heumann, SA; Hoch, U; Ivy, SN; Jacobs, JW; Lam, S; Lee, H; McDowell, RS; Oslob, JD; Purkey, HE; Romanowski, MJ; Silverman, JA; Tangonan, BT; Taverna, P; Yang, W; Yoburn, JC; Yu, CH; Zimmerman, KM; O'Brien, T; Lew, W 2-Aminobenzimidazoles as potent Aurora kinase inhibitors. Bioorg Med Chem Lett19:5158-61 (2009) [PubMed] Article
Zhong, M; Bui, M; Shen, W; Baskaran, S; Allen, DA; Elling, RA; Flanagan, WM; Fung, AD; Hanan, EJ; Harris, SO; Heumann, SA; Hoch, U; Ivy, SN; Jacobs, JW; Lam, S; Lee, H; McDowell, RS; Oslob, JD; Purkey, HE; Romanowski, MJ; Silverman, JA; Tangonan, BT; Taverna, P; Yang, W; Yoburn, JC; Yu, CH; Zimmerman, KM; O'Brien, T; Lew, W 2-Aminobenzimidazoles as potent Aurora kinase inhibitors. Bioorg Med Chem Lett19:5158-61 (2009) [PubMed] Article