| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Bifunctional epoxide hydrolase 2 |
|---|
| Ligand | BDBM50310822 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_621473 (CHEMBL1100034) |
|---|
| IC50 | 3±n/a nM |
|---|
| Citation |  Shen, HC; Ding, FX; Deng, Q; Xu, S; Chen, HS; Tong, X; Tong, V; Zhang, X; Chen, Y; Zhou, G; Pai, LY; Alonso-Galicia, M; Zhang, B; Roy, S; Tata, JR; Berger, JP; Colletti, SL Discovery of 3,3-disubstituted piperidine-derived trisubstituted ureas as highly potent soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett19:5314-20 (2009) [PubMed] Article Shen, HC; Ding, FX; Deng, Q; Xu, S; Chen, HS; Tong, X; Tong, V; Zhang, X; Chen, Y; Zhou, G; Pai, LY; Alonso-Galicia, M; Zhang, B; Roy, S; Tata, JR; Berger, JP; Colletti, SL Discovery of 3,3-disubstituted piperidine-derived trisubstituted ureas as highly potent soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett19:5314-20 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Bifunctional epoxide hydrolase 2 |
|---|
| Name: | Bifunctional epoxide hydrolase 2 |
|---|
| Synonyms: | Ephx2 | Epoxide hydrolase 2 | HYES_RAT |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 62337.15 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_1465995 |
|---|
| Residue: | 554 |
|---|
| Sequence: | MALRVAAFDLDGVLALPSIAGVLRHTEEALALPRDFLLGAFQMKFPEGPTEQLMKGKITF
SQWVPLMDESCRKSSKACGASLPENFSISEIFSQAMAARSINRPMLQAAAALKKKGFTTC
IVTNNWLDDSDKRDILAQMMCELSQHFDFLIESCQVGMIKPEPQIYKFVLDTLKAKPNEV
VFLDDFGSNLKPARDMGMVTILVRDTASALRELEKVTGTQFPEAPLPVPCSPNDVSHGYV
TVKPGIRLHFVEMGSGPAICLCHGFPESWFSWRYQIPALAQAGFRVLAIDMKGYGDSSSP
PEIEEYAMELLCEEMVTFLNKLGIPQAVFIGHDWAGVLVWNMALFHPERVRAVASLNTPL
MPPNPEVSPMEVIRSIPVFNYQLYFQEPGVAEAELEKNMSRTFKSFFRTSDDMGLLTVNK
ATEMGGILVGTPEDPKVSKITTEEEIEYYIQQFKKSGFRGPLNWYRNTERNWKWSCKALG
RKILVPALMVTAEKDIVLRPEMSKNMENWIPFLKRGHIEDCGHWTQIEKPAEVNQILIKW
LKTEIQNPSVTSKI
|
|
|
|---|
| BDBM50310822 |
|---|
| n/a |
|---|
| Name | BDBM50310822 |
|---|
| Synonyms: | CHEMBL1078754 | methyl 3-(1-(4-chlorophenylcarbamoyl)-3-phenylpiperidin-3-yl)propanoate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H25ClN2O3 |
|---|
| Mol. Mass. | 400.899 |
|---|
| SMILES | COC(=O)CCC1(CCCN(C1)C(=O)Nc1ccc(Cl)cc1)c1ccccc1 |
|---|
| Structure | 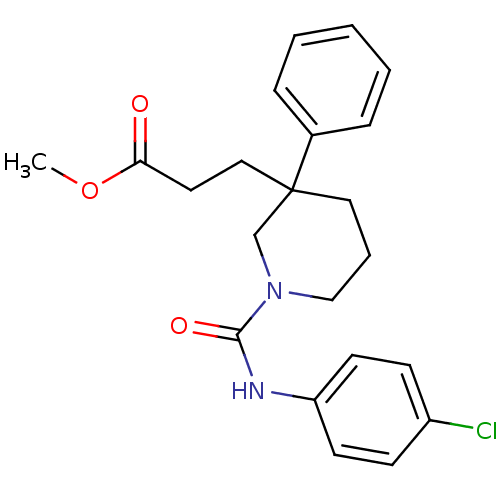
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shen, HC; Ding, FX; Deng, Q; Xu, S; Chen, HS; Tong, X; Tong, V; Zhang, X; Chen, Y; Zhou, G; Pai, LY; Alonso-Galicia, M; Zhang, B; Roy, S; Tata, JR; Berger, JP; Colletti, SL Discovery of 3,3-disubstituted piperidine-derived trisubstituted ureas as highly potent soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett19:5314-20 (2009) [PubMed] Article
Shen, HC; Ding, FX; Deng, Q; Xu, S; Chen, HS; Tong, X; Tong, V; Zhang, X; Chen, Y; Zhou, G; Pai, LY; Alonso-Galicia, M; Zhang, B; Roy, S; Tata, JR; Berger, JP; Colletti, SL Discovery of 3,3-disubstituted piperidine-derived trisubstituted ureas as highly potent soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett19:5314-20 (2009) [PubMed] Article