| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lactase/phlorizin hydrolase |
|---|
| Ligand | BDBM50163445 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_617532 (CHEMBL1099882) |
|---|
| IC50 | 400000±n/a nM |
|---|
| Citation |  Wennekes, T; Meijer, AJ; Groen, AK; Boot, RG; Groener, JE; van Eijk, M; Ottenhoff, R; Bijl, N; Ghauharali, K; Song, H; O'Shea, TJ; Liu, H; Yew, N; Copeland, D; van den Berg, RJ; van der Marel, GA; Overkleeft, HS; Aerts, JM Dual-action lipophilic iminosugar improves glycemic control in obese rodents by reduction of visceral glycosphingolipids and buffering of carbohydrate assimilation. J Med Chem53:689-98 (2010) [PubMed] Article Wennekes, T; Meijer, AJ; Groen, AK; Boot, RG; Groener, JE; van Eijk, M; Ottenhoff, R; Bijl, N; Ghauharali, K; Song, H; O'Shea, TJ; Liu, H; Yew, N; Copeland, D; van den Berg, RJ; van der Marel, GA; Overkleeft, HS; Aerts, JM Dual-action lipophilic iminosugar improves glycemic control in obese rodents by reduction of visceral glycosphingolipids and buffering of carbohydrate assimilation. J Med Chem53:689-98 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lactase/phlorizin hydrolase |
|---|
| Name: | Lactase/phlorizin hydrolase |
|---|
| Synonyms: | LCT | LPH | LPH_HUMAN | Lactase-phlorizin hydrolase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 218576.40 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1435478 |
|---|
| Residue: | 1927 |
|---|
| Sequence: | MELSWHVVFIALLSFSCWGSDWESDRNFISTAGPLTNDLLHNLSGLLGDQSSNFVAGDKD
MYVCHQPLPTFLPEYFSSLHASQITHYKVFLSWAQLLPAGSTQNPDEKTVQCYRRLLKAL
KTARLQPMVILHHQTLPASTLRRTEAFADLFADYATFAFHSFGDLVGIWFTFSDLEEVIK
ELPHQESRASQLQTLSDAHRKAYEIYHESYAFQGGKLSVVLRAEDIPELLLEPPISALAQ
DTVDFLSLDLSYECQNEASLRQKLSKLQTIEPKVKVFIFNLKLPDCPSTMKNPASLLFSL
FEAINKDQVLTIGFDINEFLSCSSSSKKSMSCSLTGSLALQPDQQQDHETTDSSPASAYQ
RIWEAFANQSRAERDAFLQDTFPEGFLWGASTGAFNVEGGWAEGGRGVSIWDPRRPLNTT
EGQATLEVASDSYHKVASDVALLCGLRAQVYKFSISWSRIFPMGHGSSPSLPGVAYYNKL
IDRLQDAGIEPMATLFHWDLPQALQDHGGWQNESVVDAFLDYAAFCFSTFGDRVKLWVTF
HEPWVMSYAGYGTGQHPPGISDPGVASFKVAHLVLKAHARTWHHYNSHHRPQQQGHVGIV
LNSDWAEPLSPERPEDLRASERFLHFMLGWFAHPVFVDGDYPATLRTQIQQMNRQCSHPV
AQLPEFTEAEKQLLKGSADFLGLSHYTSRLISNAPQNTCIPSYDTIGGFSQHVNHVWPQT
SSSWIRVVPWGIRRLLQFVSLEYTRGKVPIYLAGNGMPIGESENLFDDSLRVDYFNQYIN
EVLKAIKEDSVDVRSYIARSLIDGFEGPSGYSQRFGLHHVNFSDSSKSRTPRKSAYFFTS
IIEKNGFLTKGAKRLLPPNTVNLPSKVRAFTFPSEVPSKAKVVWEKFSSQPKFERDLFYH
GTFRDDFLWGVSSSAYQIEGAWDADGKGPSIWDNFTHTPGSNVKDNATGDIACDSYHQLD
ADLNMLRALKVKAYRFSISWSRIFPTGRNSSINSHGVDYYNRLINGLVASNIFPMVTLFH
WDLPQALQDIGGWENPALIDLFDSYADFCFQTFGDRVKFWMTFNEPMYLAWLGYGSGEFP
PGVKDPGWAPYRIAHAVIKAHARVYHTYDEKYRQEQKGVISLSLSTHWAEPKSPGVPRDV
EAADRMLQFSLGWFAHPIFRNGDYPDTMKWKVGNRSELQHLATSRLPSFTEEEKRFIRAT
ADVFCLNTYYSRIVQHKTPRLNPPSYEDDQEMAEEEDPSWPSTAMNRAAPWGTRRLLNWI
KEEYGDIPIYITENGVGLTNPNTEDTDRIFYHKTYINEALKAYRLDGIDLRGYVAWSLMD
NFEWLNGYTVKFGLYHVDFNNTNRPRTARASARYYTEVITNNGMPLAREDEFLYGRFPEG
FIWSAASAAYQIEGAWRADGKGLSIWDTFSHTPLRVENDAIGDVACDSYHKIAEDLVTLQ
NLGVSHYRFSISWSRILPDGTTRYINEAGLNYYVRLIDTLLAASIQPQVTIYHWDLPQTL
QDVGGWENETIVQRFKEYADVLFQRLGDKVKFWITLNEPFVIAYQGYGYGTAAPGVSNRP
GTAPYIVGHNLIKAHAEAWHLYNDVYRASQGGVISITISSDWAEPRDPSNQEDVEAARRY
VQFMGGWFAHPIFKNGDYNEVMKTRIRDRSLAAGLNKSRLPEFTESEKRRINGTYDFFGF
NHYTTVLAYNLNYATAISSFDADRGVASIADRSWPDSGSFWLKMTPFGFRRILNWLKEEY
NDPPIYVTENGVSQREETDLNDTARIYYLRTYINEALKAVQDKVDLRGYTVWSAMDNFEW
ATGFSERFGLHFVNYSDPSLPRIPKASAKFYASVVRCNGFPDPATGPHACLHQPDAGPTI
SPVRQEEVQFLGLMLGTTEAQTALYVLFSLVLLGVCGLAFLSYKYCKRSKQGKTQRSQQE
LSPVSSF
|
|
|
|---|
| BDBM50163445 |
|---|
| n/a |
|---|
| Name | BDBM50163445 |
|---|
| Synonyms: | (2S,3S,4R,5S)-2-Hydroxymethyl-piperidine-3,4,5-triol | CHEMBL179409 | L-altro-1-Deoxynojirimycin |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C6H13NO4 |
|---|
| Mol. Mass. | 163.1717 |
|---|
| SMILES | OC[C@@H]1NC[C@H](O)[C@@H](O)[C@H]1O |
|---|
| Structure | 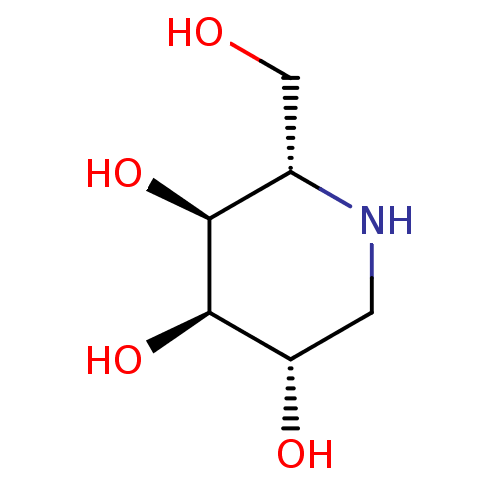
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wennekes, T; Meijer, AJ; Groen, AK; Boot, RG; Groener, JE; van Eijk, M; Ottenhoff, R; Bijl, N; Ghauharali, K; Song, H; O'Shea, TJ; Liu, H; Yew, N; Copeland, D; van den Berg, RJ; van der Marel, GA; Overkleeft, HS; Aerts, JM Dual-action lipophilic iminosugar improves glycemic control in obese rodents by reduction of visceral glycosphingolipids and buffering of carbohydrate assimilation. J Med Chem53:689-98 (2010) [PubMed] Article
Wennekes, T; Meijer, AJ; Groen, AK; Boot, RG; Groener, JE; van Eijk, M; Ottenhoff, R; Bijl, N; Ghauharali, K; Song, H; O'Shea, TJ; Liu, H; Yew, N; Copeland, D; van den Berg, RJ; van der Marel, GA; Overkleeft, HS; Aerts, JM Dual-action lipophilic iminosugar improves glycemic control in obese rodents by reduction of visceral glycosphingolipids and buffering of carbohydrate assimilation. J Med Chem53:689-98 (2010) [PubMed] Article