

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Receptor tyrosine-protein kinase erbB-2 | ||
| Ligand | BDBM50313648 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_614502 (CHEMBL1111475) | ||
| IC50 | 81.0±n/a nM | ||
| Citation |  Morphy, R Selectively nonselective kinase inhibition: striking the right balance. J Med Chem53:1413-37 (2010) [PubMed] Article Morphy, R Selectively nonselective kinase inhibition: striking the right balance. J Med Chem53:1413-37 (2010) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Receptor tyrosine-protein kinase erbB-2 | |||
| Name: | Receptor tyrosine-protein kinase erbB-2 | ||
| Synonyms: | 2.7.10.1 | C-erbB-2 | CD_antigen=CD340 | ERBB2 | ERBB2_HUMAN | ErbB-2/ErbB-3 heterodimer | FASN/HER2 | HER-2 Substrate | HER2 | MLN 19 | MLN19 | Metastatic lymph node gene 19 protein | NEU | NGL | Proto-oncogene Neu | Proto-oncogene c-ErbB-2 | Tyrosine kinase-type cell surface receptor HER2 | p185erbB2 | ||
| Type: | n/a | ||
| Mol. Mass.: | 137894.47 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P04626 | ||
| Residue: | 1255 | ||
| Sequence: |
| ||
| BDBM50313648 | |||
| n/a | |||
| Name | BDBM50313648 | ||
| Synonyms: | CHEMBL2374458 | trans-3-(2-(2-methoxybenzyl)-1H-benzo[d]imidazol-6-yl)-1-(4-(1-methylpiperidin-4-yl)cyclohexyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine | ||
| Type | Small organic molecule | ||
| Emp. Form. | C32H38N8O | ||
| Mol. Mass. | 550.6971 | ||
| SMILES | COc1ccccc1Cc1nc2ccc(cc2[nH]1)-c1nn([C@H]2CC[C@@H](CC2)C2CCN(C)CC2)c2ncnc(N)c12 |r,wU:21.23,wD:24.30,(12.06,2.98,;13.6,2.98,;14.37,4.32,;13.6,5.65,;14.37,6.99,;15.91,6.99,;16.68,5.65,;15.91,4.32,;16.68,2.98,;18.22,2.98,;19.13,4.23,;20.59,3.75,;21.92,4.52,;23.26,3.75,;23.26,2.21,;21.92,1.44,;20.59,2.21,;19.13,1.74,;24.59,1.44,;24.75,-.09,;26.26,-.41,;26.89,-1.81,;28.42,-1.97,;29.04,-3.38,;28.14,-4.63,;26.61,-4.47,;25.98,-3.06,;28.76,-6.03,;30.3,-6.2,;30.92,-7.6,;30.02,-8.85,;30.64,-10.25,;28.49,-8.69,;27.86,-7.28,;27.03,.93,;28.53,1.25,;29.01,2.71,;27.98,3.86,;26.47,3.54,;25.44,4.68,;26,2.07,)| | ||
| Structure | 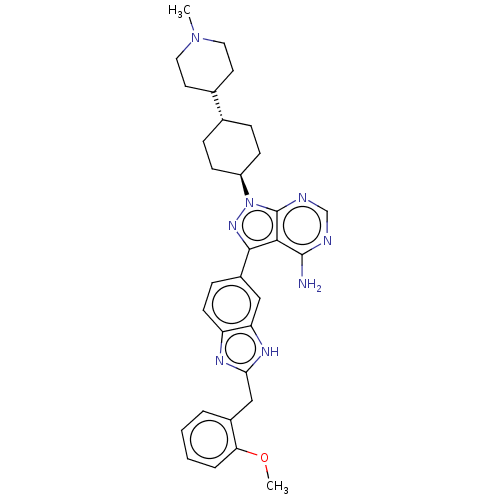
| ||