| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetyl-CoA carboxylase 2 |
|---|
| Ligand | BDBM50314888 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_627704 (CHEMBL1115284) |
|---|
| IC50 | 11±n/a nM |
|---|
| Citation |  Corbett, JW; Freeman-Cook, KD; Elliott, R; Vajdos, F; Rajamohan, F; Kohls, D; Marr, E; Zhang, H; Tong, L; Tu, M; Murdande, S; Doran, SD; Houser, JA; Song, W; Jones, CJ; Coffey, SB; Buzon, L; Minich, ML; Dirico, KJ; Tapley, S; McPherson, RK; Sugarman, E; Harwood, HJ; Esler, W Discovery of small molecule isozyme non-specific inhibitors of mammalian acetyl-CoA carboxylase 1 and 2. Bioorg Med Chem Lett20:2383-8 (2010) [PubMed] Article Corbett, JW; Freeman-Cook, KD; Elliott, R; Vajdos, F; Rajamohan, F; Kohls, D; Marr, E; Zhang, H; Tong, L; Tu, M; Murdande, S; Doran, SD; Houser, JA; Song, W; Jones, CJ; Coffey, SB; Buzon, L; Minich, ML; Dirico, KJ; Tapley, S; McPherson, RK; Sugarman, E; Harwood, HJ; Esler, W Discovery of small molecule isozyme non-specific inhibitors of mammalian acetyl-CoA carboxylase 1 and 2. Bioorg Med Chem Lett20:2383-8 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetyl-CoA carboxylase 2 |
|---|
| Name: | Acetyl-CoA carboxylase 2 |
|---|
| Synonyms: | ACACB | ACACB_HUMAN | ACC-beta | ACC2 | ACCB | Acetyl-CoA carboxylase | Acetyl-CoA carboxylase 2 | Acetyl-CoA carboxylase 2 (ACC) | Acetyl-CoA carboxylase 2 (ACC2) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 276535.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O00763 |
|---|
| Residue: | 2458 |
|---|
| Sequence: | MVLLLCLSCLIFSCLTFSWLKIWGKMTDSKPITKSKSEANLIPSQEPFPASDNSGETPQR
NGEGHTLPKTPSQAEPASHKGPKDAGRRRNSLPPSHQKPPRNPLSSSDAAPSPELQANGT
GTQGLEATDTNGLSSSARPQGQQAGSPSKEDKKQANIKRQLMTNFILGSFDDYSSDEDSV
AGSSRESTRKGSRASLGALSLEAYLTTGEAETRVPTMRPSMSGLHLVKRGREHKKLDLHR
DFTVASPAEFVTRFGGDRVIEKVLIANNGIAAVKCMRSIRRWAYEMFRNERAIRFVVMVT
PEDLKANAEYIKMADHYVPVPGGPNNNNYANVELIVDIAKRIPVQAVWAGWGHASENPKL
PELLCKNGVAFLGPPSEAMWALGDKIASTVVAQTLQVPTLPWSGSGLTVEWTEDDLQQGK
RISVPEDVYDKGCVKDVDEGLEAAERIGFPLMIKASEGGGGKGIRKAESAEDFPILFRQV
QSEIPGSPIFLMKLAQHARHLEVQILADQYGNAVSLFGRDCSIQRRHQKIVEEAPATIAP
LAIFEFMEQCAIRLAKTVGYVSAGTVEYLYSQDGSFHFLELNPRLQVEHPCTEMIADVNL
PAAQLQIAMGVPLHRLKDIRLLYGESPWGVTPISFETPSNPPLARGHVIAARITSENPDE
GFKPSSGTVQELNFRSSKNVWGYFSVAATGGLHEFADSQFGHCFSWGENREEAISNMVVA
LKELSIRGDFRTTVEYLINLLETESFQNNDIDTGWLDYLIAEKVQAEKPDIMLGVVCGAL
NVADAMFRTCMTDFLHSLERGQVLPADSLLNLVDVELIYGGVKYILKVARQSLTMFVLIM
NGCHIEIDAHRLNDGGLLLSYNGNSYTTYMKEEVDSYRITIGNKTCVFEKENDPTVLRSP
SAGKLTQYTVEDGGHVEAGSSYAEMEVMKMIMTLNVQERGRVKYIKRPGAVLEAGCVVAR
LELDDPSKVHPAEPFTGELPAQQTLPILGEKLHQVFHSVLENLTNVMSGFCLPEPVFSIK
LKEWVQKLMMTLRHPSLPLLELQEIMTSVAGRIPAPVEKSVRRVMAQYASNITSVLCQFP
SQQIATILDCHAATLQRKADREVFFINTQSIVQLVQRYRSGIRGYMKTVVLDLLRRYLRV
EHHFQQAHYDKCVINLREQFKPDMSQVLDCIFSHAQVAKKNQLVIMLIDELCGPDPSLSD
ELISILNELTQLSKSEHCKVALRARQILIASHLPSYELRHNQVESIFLSAIDMYGHQFCP
ENLKKLILSETTIFDVLPTFFYHANKVVCMASLEVYVRRGYIAYELNSLQHRQLPDGTCV
VEFQFMLPSSHPNRMTVPISITNPDLLRHSTELFMDSGFSPLCQRMGAMVAFRRFEDFTR
NFDEVISCFANVPKDTPLFSEARTSLYSEDDCKSLREEPIHILNVSIQCADHLEDEALVP
ILRTFVQSKKNILVDYGLRRITFLIAQEKEFPKFFTFRARDEFAEDRIYRHLEPALAFQL
ELNRMRNFDLTAVPCANHKMHLYLGAAKVKEGVEVTDHRFFIRAIIRHSDLITKEASFEY
LQNEGERLLLEAMDELEVAFNNTSVRTDCNHIFLNFVPTVIMDPFKIEESVRYMVMRYGS
RLWKLRVLQAEVKINIRQTTTGSAVPIRLFITNESGYYLDISLYKEVTDSRSGNIMFHSF
GNKQGPQHGMLINTPYVTKDLLQAKRFQAQTLGTTYIYDFPEMFRQALFKLWGSPDKYPK
DILTYTELVLDSQGQLVEMNRLPGGNEVGMVAFKMRFKTQEYPEGRDVIVIGNDITFRIG
SFGPGEDLLYLRASEMARAEGIPKIYVAANSGARIGMAEEIKHMFHVAWVDPEDPHKGFK
YLYLTPQDYTRISSLNSVHCKHIEEGGESRYMITDIIGKDDGLGVENLRGSGMIAGESSL
AYEEIVTISLVTCRAIGIGAYLVRLGQRVIQVENSHIILTGASALNKVLGREVYTSNNQL
GGVQIMHYNGVSHITVPDDFEGVYTILEWLSYMPKDNHSPVPIITPTDPIDREIEFLPSR
APYDPRWMLAGRPHPTLKGTWQSGFFDHGSFKEIMAPWAQTVVTGRARLGGIPVGVIAVE
TRTVEVAVPADPANLDSEAKIIQQAGQVWFPDSAYKTAQAVKDFNREKLPLMIFANWRGF
SGGMKDMYDQVLKFGAYIVDGLRQYKQPILIYIPPYAELRGGSWVVIDATINPLCIEMYA
DKESRGGVLEPEGTVEIKFRKKDLIKSMRRIDPAYKKLMEQLGEPDLSDKDRKDLEGRLK
AREDLLLPIYHQVAVQFADFHDTPGRMLEKGVISDILEWKTARTFLYWRLRRLLLEDQVK
QEILQASGELSHVHIQSMLRRWFVETEGAVKAYLWDNNQVVVQWLEQHWQAGDGPRSTIR
ENITYLKHDSVLKTIRGLVEENPEVAVDCVIYLSQHISPAERAQVVHLLSTMDSPAST
|
|
|
|---|
| BDBM50314888 |
|---|
| n/a |
|---|
| Name | BDBM50314888 |
|---|
| Synonyms: | 1'-(3,7-dimethyl-1H-indazole-5-carbonyl)-5-methoxyspiro[chroman-2,4'-piperidin]-4-one | CHEMBL1092308 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H25N3O4 |
|---|
| Mol. Mass. | 419.473 |
|---|
| SMILES | COc1cccc2OC3(CCN(CC3)C(=O)c3cc(C)c4[nH]nc(C)c4c3)CC(=O)c12 |
|---|
| Structure | 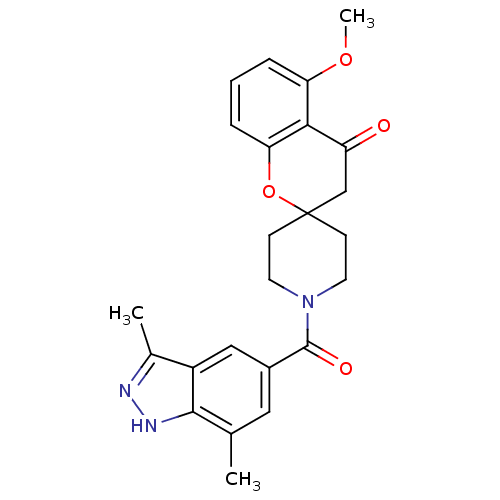
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Corbett, JW; Freeman-Cook, KD; Elliott, R; Vajdos, F; Rajamohan, F; Kohls, D; Marr, E; Zhang, H; Tong, L; Tu, M; Murdande, S; Doran, SD; Houser, JA; Song, W; Jones, CJ; Coffey, SB; Buzon, L; Minich, ML; Dirico, KJ; Tapley, S; McPherson, RK; Sugarman, E; Harwood, HJ; Esler, W Discovery of small molecule isozyme non-specific inhibitors of mammalian acetyl-CoA carboxylase 1 and 2. Bioorg Med Chem Lett20:2383-8 (2010) [PubMed] Article
Corbett, JW; Freeman-Cook, KD; Elliott, R; Vajdos, F; Rajamohan, F; Kohls, D; Marr, E; Zhang, H; Tong, L; Tu, M; Murdande, S; Doran, SD; Houser, JA; Song, W; Jones, CJ; Coffey, SB; Buzon, L; Minich, ML; Dirico, KJ; Tapley, S; McPherson, RK; Sugarman, E; Harwood, HJ; Esler, W Discovery of small molecule isozyme non-specific inhibitors of mammalian acetyl-CoA carboxylase 1 and 2. Bioorg Med Chem Lett20:2383-8 (2010) [PubMed] Article