| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50315069 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_624681 (CHEMBL1112473) |
|---|
| IC50 | >30000±n/a nM |
|---|
| Citation |  Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Yu, K; Zask, A 2-Arylureidophenyl-4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)triazines as highly potent and selective ATP competitive mTOR inhibitors: optimization of human microsomal stability. Bioorg Med Chem Lett20:2648-53 (2010) [PubMed] Article Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Yu, K; Zask, A 2-Arylureidophenyl-4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)triazines as highly potent and selective ATP competitive mTOR inhibitors: optimization of human microsomal stability. Bioorg Med Chem Lett20:2648-53 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50315069 |
|---|
| n/a |
|---|
| Name | BDBM50315069 |
|---|
| Synonyms: | 1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-(tetrahydro-2H-pyran-4-yl)-1,3,5-triazin-2-yl)phenyl)-3-(4-(4-isopropylpiperazine-1-carbonyl)phenyl)urea | CHEMBL1091356 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C35H44N8O4 |
|---|
| Mol. Mass. | 640.7751 |
|---|
| SMILES | CC(C)N1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2C3CCC2COC3)C2CCOCC2)cc1 |
|---|
| Structure | 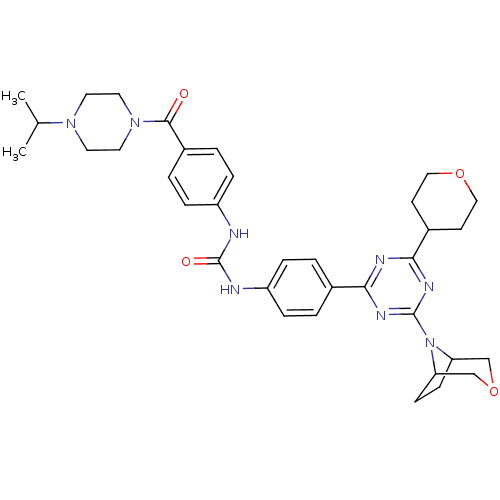
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Yu, K; Zask, A 2-Arylureidophenyl-4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)triazines as highly potent and selective ATP competitive mTOR inhibitors: optimization of human microsomal stability. Bioorg Med Chem Lett20:2648-53 (2010) [PubMed] Article
Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Yu, K; Zask, A 2-Arylureidophenyl-4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)triazines as highly potent and selective ATP competitive mTOR inhibitors: optimization of human microsomal stability. Bioorg Med Chem Lett20:2648-53 (2010) [PubMed] Article