| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aurora kinase B |
|---|
| Ligand | BDBM50316253 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_628512 (CHEMBL1104634) |
|---|
| IC50 | 45100±n/a nM |
|---|
| Citation |  Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem52:8010-24 (2009) [PubMed] Article Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem52:8010-24 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aurora kinase B |
|---|
| Name: | Aurora kinase B |
|---|
| Synonyms: | AIK2 | AIM-1 | AIM1 | AIRK2 | ARK2 | AURKB | AURKB_HUMAN | Aurora B kinase (aurB) | Aurora B-INCENP | Aurora kinase 2 | Aurora kinase B (AURKB) | Aurora-related kinase 2 | STK-1 | STK1 | STK12 | STK5 | Serine/threonine-protein kinase aurora B |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 39327.72 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q96GD4 |
|---|
| Residue: | 344 |
|---|
| Sequence: | MAQKENSYPWPYGRQTAPSGLSTLPQRVLRKEPVTPSALVLMSRSNVQPTAAPGQKVMEN
SSGTPDILTRHFTIDDFEIGRPLGKGKFGNVYLAREKKSHFIVALKVLFKSQIEKEGVEH
QLRREIEIQAHLHHPNILRLYNYFYDRRRIYLILEYAPRGELYKELQKSCTFDEQRTATI
MEELADALMYCHGKKVIHRDIKPENLLLGLKGELKIADFGWSVHAPSLRRKTMCGTLDYL
PPEMIEGRMHNEKVDLWCIGVLCYELLVGNPPFESASHNETYRRIVKVDLKFPASVPMGA
QDLISKLLRHNPSERLPLAQVSAHPWVRANSRRVLPPSALQSVA
|
|
|
|---|
| BDBM50316253 |
|---|
| n/a |
|---|
| Name | BDBM50316253 |
|---|
| Synonyms: | CHEMBL1095627 | tert-Butyl 4-(6-(4-aminophenyl)-4-morpholino-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carboxylate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H33N7O3 |
|---|
| Mol. Mass. | 479.5746 |
|---|
| SMILES | CC(C)(C)OC(=O)N1CCC(CC1)n1ncc2c(nc(nc12)-c1ccc(N)cc1)N1CCOCC1 |
|---|
| Structure | 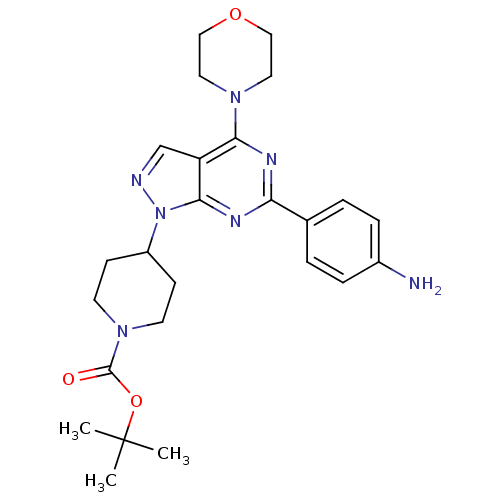
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem52:8010-24 (2009) [PubMed] Article
Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem52:8010-24 (2009) [PubMed] Article