| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sphingosine 1-phosphate receptor 3 |
|---|
| Ligand | BDBM50316733 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_632482 (CHEMBL1108346) |
|---|
| EC50 | 525±n/a nM |
|---|
| Citation |  Bolli, MH; Abele, S; Binkert, C; Bravo, R; Buchmann, S; Bur, D; Gatfield, J; Hess, P; Kohl, C; Mangold, C; Mathys, B; Menyhart, K; Müller, C; Nayler, O; Scherz, M; Schmidt, G; Sippel, V; Steiner, B; Strasser, D; Treiber, A; Weller, T 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem53:4198-211 (2010) [PubMed] Article Bolli, MH; Abele, S; Binkert, C; Bravo, R; Buchmann, S; Bur, D; Gatfield, J; Hess, P; Kohl, C; Mangold, C; Mathys, B; Menyhart, K; Müller, C; Nayler, O; Scherz, M; Schmidt, G; Sippel, V; Steiner, B; Strasser, D; Treiber, A; Weller, T 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem53:4198-211 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sphingosine 1-phosphate receptor 3 |
|---|
| Name: | Sphingosine 1-phosphate receptor 3 |
|---|
| Synonyms: | C9orf108 | C9orf47 | EDG3 | Endothelial differentiation G-protein coupled receptor 3 | S1P receptor 3 | S1P receptor Edg-3 | S1P3 | S1PR3 | S1PR3_HUMAN | Sphingosine 1-phosphate receptor | Sphingosine 1-phosphate receptor 3 (S1P3) | Sphingosine 1-phosphate receptor Edg-3 |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 42278.13 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q99500 |
|---|
| Residue: | 378 |
|---|
| Sequence: | MATALPPRLQPVRGNETLREHYQYVGKLAGRLKEASEGSTLTTVLFLVICSFIVLENLMV
LIAIWKNNKFHNRMYFFIGNLALCDLLAGIAYKVNILMSGKKTFSLSPTVWFLREGSMFV
ALGASTCSLLAIAIERHLTMIKMRPYDANKRHRVFLLIGMCWLIAFTLGALPILGWNCLH
NLPDCSTILPLYSKKYIAFCISIFTAILVTIVILYARIYFLVKSSSRKVANHNNSERSMA
LLRTVVIVVSVFIACWSPLFILFLIDVACRVQACPILFKAQWFIVLAVLNSAMNPVIYTL
ASKEMRRAFFRLVCNCLVRGRGARASPIQPALDPSRSKSSSSNNSSHSPKVKEDLPHTAP
SSCIMDKNAALQNGIFCN
|
|
|
|---|
| BDBM50316733 |
|---|
| n/a |
|---|
| Name | BDBM50316733 |
|---|
| Synonyms: | (Z,Z)-5-(3-Chloro-4-hydroxy-benzylidene)-3-(2,4-dimethoxy-phenyl)-2-isopropylimino-thiazolidin-4-one | CHEMBL1094246 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H21ClN2O4S |
|---|
| Mol. Mass. | 432.92 |
|---|
| SMILES | COc1ccc(N2\C(S\C(=C/c3ccc(O)c(Cl)c3)C2=O)=N\C(C)C)c(OC)c1 |
|---|
| Structure | 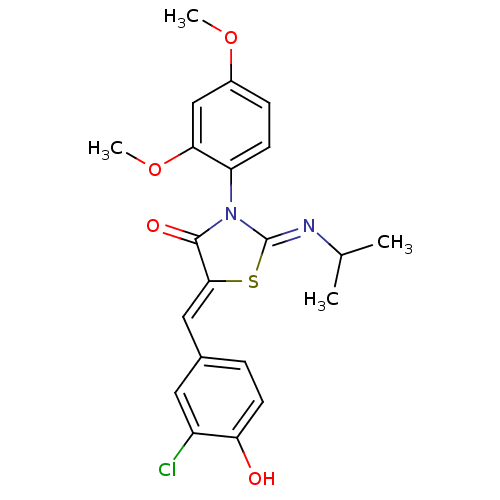
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bolli, MH; Abele, S; Binkert, C; Bravo, R; Buchmann, S; Bur, D; Gatfield, J; Hess, P; Kohl, C; Mangold, C; Mathys, B; Menyhart, K; Müller, C; Nayler, O; Scherz, M; Schmidt, G; Sippel, V; Steiner, B; Strasser, D; Treiber, A; Weller, T 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem53:4198-211 (2010) [PubMed] Article
Bolli, MH; Abele, S; Binkert, C; Bravo, R; Buchmann, S; Bur, D; Gatfield, J; Hess, P; Kohl, C; Mangold, C; Mathys, B; Menyhart, K; Müller, C; Nayler, O; Scherz, M; Schmidt, G; Sippel, V; Steiner, B; Strasser, D; Treiber, A; Weller, T 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem53:4198-211 (2010) [PubMed] Article