| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform |
|---|
| Ligand | BDBM13535 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_655876 (CHEMBL1244920) |
|---|
| Kd | >10000±n/a nM |
|---|
| Citation |  Zarrinkar, PP; Gunawardane, RN; Cramer, MD; Gardner, MF; Brigham, D; Belli, B; Karaman, MW; Pratz, KW; Pallares, G; Chao, Q; Sprankle, KG; Patel, HK; Levis, M; Armstrong, RC; James, J; Bhagwat, SS AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood114:2984-92 (2009) [PubMed] Article Zarrinkar, PP; Gunawardane, RN; Cramer, MD; Gardner, MF; Brigham, D; Belli, B; Karaman, MW; Pratz, KW; Pallares, G; Chao, Q; Sprankle, KG; Patel, HK; Levis, M; Armstrong, RC; James, J; Bhagwat, SS AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood114:2984-92 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform |
|---|
| Name: | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform |
|---|
| Synonyms: | PI3-kinase p110 subunit gamma | PI3-kinase subunit p120-gamma | PI3Kgamma | PIK3CG | PK3CG_HUMAN | Phosphatidylinositol 4,5-biphosphate 3-kinase catalytic subunit gamma (PIK3CG) | Phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase 110 kDa catalytic subunit gamma (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma (PI3Kgamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3Kgamma) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Phosphoinositide 3-Kinase (PI3K), gamma Chain A | Phosphoinositide 3-kinases gamma (PI3K gamma) | Phosphoinositide-3-kinase (PI3K gamma) | p120-PI3K |
|---|
| Type: | Enzyme Subunit |
|---|
| Mol. Mass.: | 126470.30 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P48736 |
|---|
| Residue: | 1102 |
|---|
| Sequence: | MELENYKQPVVLREDNCRRRRRMKPRSAAASLSSMELIPIEFVLPTSQRKCKSPETALLH
VAGHGNVEQMKAQVWLRALETSVAADFYHRLGPHHFLLLYQKKGQWYEIYDKYQVVQTLD
CLRYWKATHRSPGQIHLVQRHPPSEESQAFQRQLTALIGYDVTDVSNVHDDELEFTRRGL
VTPRMAEVASRDPKLYAMHPWVTSKPLPEYLWKKIANNCIFIVIHRSTTSQTIKVSPDDT
PGAILQSFFTKMAKKKSLMDIPESQSEQDFVLRVCGRDEYLVGETPIKNFQWVRHCLKNG
EEIHVVLDTPPDPALDEVRKEEWPLVDDCTGVTGYHEQLTIHGKDHESVFTVSLWDCDRK
FRVKIRGIDIPVLPRNTDLTVFVEANIQHGQQVLCQRRTSPKPFTEEVLWNVWLEFSIKI
KDLPKGALLNLQIYCGKAPALSSKASAESPSSESKGKVQLLYYVNLLLIDHRFLLRRGEY
VLHMWQISGKGEDQGSFNADKLTSATNPDKENSMSISILLDNYCHPIALPKHQPTPDPEG
DRVRAEMPNQLRKQLEAIIATDPLNPLTAEDKELLWHFRYESLKHPKAYPKLFSSVKWGQ
QEIVAKTYQLLARREVWDQSALDVGLTMQLLDCNFSDENVRAIAVQKLESLEDDDVLHYL
LQLVQAVKFEPYHDSALARFLLKRGLRNKRIGHFLFWFLRSEIAQSRHYQQRFAVILEAY
LRGCGTAMLHDFTQQVQVIEMLQKVTLDIKSLSAEKYDVSSQVISQLKQKLENLQNSQLP
ESFRVPYDPGLKAGALAIEKCKVMASKKKPLWLEFKCADPTALSNETIGIIFKHGDDLRQ
DMLILQILRIMESIWETESLDLCLLPYGCISTGDKIGMIEIVKDATTIAKIQQSTVGNTG
AFKDEVLNHWLKEKSPTEEKFQAAVERFVYSCAGYCVATFVLGIGDRHNDNIMITETGNL
FHIDFGHILGNYKSFLGINKERVPFVLTPDFLFVMGTSGKKTSPHFQKFQDICVKAYLAL
RHHTNLLIILFSMMLMTGMPQLTSKEDIEYIRDALTVGKNEEDAKKYFLDQIEVCRDKGW
TVQFNWFLHLVLGIKQGEKHSA
|
|
|
|---|
| BDBM13535 |
|---|
| n/a |
|---|
| Name | BDBM13535 |
|---|
| Synonyms: | 4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl]-N-(4-propan-2-yloxyphenyl)piperazine-1-carboxamide | 4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl]-N-[4-(1-methylethoxy)phenyl]piperazine-1-carboxamide | 4-[6-methoxy-7-[3-(1-piperidinyl)propoxy]-4-quinazolinyl]-N-(4-propan-2-yloxyphenyl)-1-piperazinecarboxamide | 4-{6-methoxy-7-[3-(piperidin-1-yl)propoxy]quinazolin-4-yl}-N-[4-(propan-2-yloxy)phenyl]piperazine-1-carboxamide | CHEMBL124660 | MLN-518 | MLN518 | N-(4-isopropoxyphenyl)-4-[6-methoxy-7-(3-piperidinopropoxy)quinazolin-4-yl]piperazine-1-carboxamide | cid_3038522 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H42N6O4 |
|---|
| Mol. Mass. | 562.703 |
|---|
| SMILES | COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 |
|---|
| Structure | 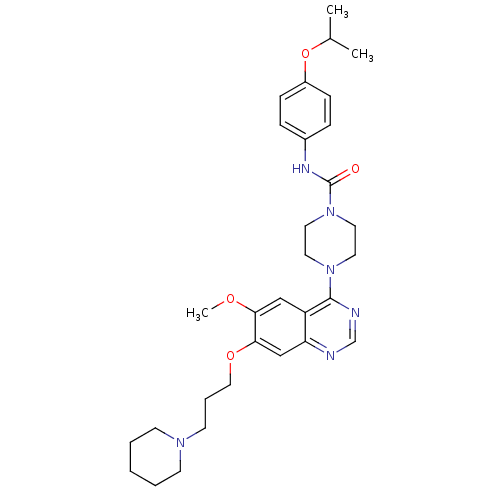
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zarrinkar, PP; Gunawardane, RN; Cramer, MD; Gardner, MF; Brigham, D; Belli, B; Karaman, MW; Pratz, KW; Pallares, G; Chao, Q; Sprankle, KG; Patel, HK; Levis, M; Armstrong, RC; James, J; Bhagwat, SS AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood114:2984-92 (2009) [PubMed] Article
Zarrinkar, PP; Gunawardane, RN; Cramer, MD; Gardner, MF; Brigham, D; Belli, B; Karaman, MW; Pratz, KW; Pallares, G; Chao, Q; Sprankle, KG; Patel, HK; Levis, M; Armstrong, RC; James, J; Bhagwat, SS AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood114:2984-92 (2009) [PubMed] Article