| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta |
|---|
| Ligand | BDBM50328584 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_665729 (CHEMBL1261698) |
|---|
| IC50 | 549±n/a nM |
|---|
| Citation |  Liu, Q; Chang, JW; Wang, J; Kang, SA; Thoreen, CC; Markhard, A; Hur, W; Zhang, J; Sim, T; Sabatini, DM; Gray, NS Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem53:7146-55 (2010) [PubMed] Article Liu, Q; Chang, JW; Wang, J; Kang, SA; Thoreen, CC; Markhard, A; Hur, W; Zhang, J; Sim, T; Sabatini, DM; Gray, NS Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem53:7146-55 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta |
|---|
| Name: | Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta |
|---|
| Synonyms: | C2-PI3K | P3C2B_HUMAN | PI3K-C2beta | PIK3C2B | Phosphatidylinositol 4-phosphate 3-kinase C2 beta (PIK3C2B) | Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing beta polypeptide | Phosphoinositide 3-Kinase (PI3K), C2beta | Phosphoinositide 3-Kinase-C2-beta | PtdIns-3-kinase C2 beta |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 184784.86 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O00750 |
|---|
| Residue: | 1634 |
|---|
| Sequence: | MSSTQGNGEHWKSLESVGISRKELAMAEALQMEYDALSRLRHDKEENRAKQNADPSLISW
DEPGVDFYSKPAGRRTDLKLLRGLSGSDPTLNYNSLSPQEGPPNHSTSQGPQPGSDPWPK
GSLSGDYLYIFDGSDGGVSSSPGPGDIEGSCKKLSPPPLPPRASIWDTPPLPPRKGSPSS
SKISQPSDINTFSLVEQLPGKLLEHRILEEEEVLGGGGQGRLLGSVDYDGINDAITRLNL
KSTYDAEMLRDATRGWKEGRGPLDFSKDTSGKPVARSKTMPPQVPPRTYASRYGNRKNAT
PGKNRRISAAPVGSRPHTVANGHELFEVSEERDEEVAAFCHMLDILRSGSDIQDYFLTGY
VWSAVTPSPEHLGDEVNLKVTVLCDRLQEALTFTCNCSSTVDLLIYQTLCYTHDDLRNVD
VGDFVLKPCGLEEFLQNKHALGSHEYIQYCRKFDIDIRLQLMEQKVVRSDLARTVNDDQS
PSTLNYLVHLQERPVKQTISRQALSLLFDTYHNEVDAFLLADGDFPLKADRVVQSVKAIC
NALAAVETPEITSALNQLPPCPSRMQPKIQKDPSVLAVRENREKVVEALTAAILDLVELY
CNTFNADFQTAVPGSRKHDLVQEACHFARSLAFTVYATHRIPIIWATSYEDFYLSCSLSH
GGKELCSPLQTRRAHFSKYLFHLIVWDQQICFPVQVNRLPRETLLCATLYALPIPPPGSS
SEANKQRRVPEALGWVTTPLFNFRQVLTCGRKLLGLWPATQENPSARWSAPNFHQPDSVI
LQIDFPTSAFDIKFTSPPGDKFSPRYEFGSLREEDQRKLKDIMQKESLYWLTDADKKRLW
EKRYYCHSEVSSLPLVLASAPSWEWACLPDIYVLLKQWTHMNHQDALGLLHATFPDQEVR
RMAVQWIGSLSDAELLDYLPQLVQALKYECYLDSPLVRFLLKRAVSDLRVTHYFFWLLKD
GLKDSQFSIRYQYLLAALLCCCGKGLREEFNRQCWLVNALAKLAQQVREAAPSARQGILR
TGLEEVKQFFALNGSCRLPLSPSLLVKGIVPRDCSYFNSNAVPLKLSFQNVDPLGENIRV
IFKCGDDLRQDMLTLQMIRIMSKIWVQEGLDMRMVIFRCFSTGRGRGMVEMIPNAETLRK
IQVEHGVTGSFKDRPLADWLQKHNPGEDEYEKAVENFIYSCAGCCVATYVLGICDRHNDN
IMLKTTGHMFHIDFGRFLGHAQMFGNIKRDRAPFVFTSDMAYVINGGDKPSSRFHDFVDL
CCQAYNLIRKHTHLFLNLLGLMLSCGIPELSDLEDLKYVYDALRPQDTEANATTYFTRLI
ESSLGSVATKLNFFIHNLAQMKFTGSDDRLTLSFASRTHTLKSSGRISDVFLCRHEKIFH
PNKGYIYVVKVMRENTHEATYIQRTFEEFQELHNKLRLLFPSSHLPSFPSRFVIGRSRGE
AVAERRREELNGYIWHLIHAPPEVAECDLVYTFFHPLPRDEKAMGTSPAPKSSDGTWARP
VGKVGGEVKLSISYKNNKLFIMVMHIRGLQLLQDGNDPDPYVKIYLLPDPQKTTKRKTKV
ARKTCNPTYNEMLVYDGIPKGDLQQRELQLSVLSEQGFWENVLLGEVNIRLRELDLAQEK
TGWFALGSRSHGTL
|
|
|
|---|
| BDBM50328584 |
|---|
| n/a |
|---|
| Name | BDBM50328584 |
|---|
| Synonyms: | 1-(4-(4-Propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one | CHEMBL1256459 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C35H28F3N5O2 |
|---|
| Mol. Mass. | 607.6243 |
|---|
| SMILES | CCC(=O)N1CCN(CC1)c1ccc(cc1C(F)(F)F)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cnc2ccccc2c1 |
|---|
| Structure | 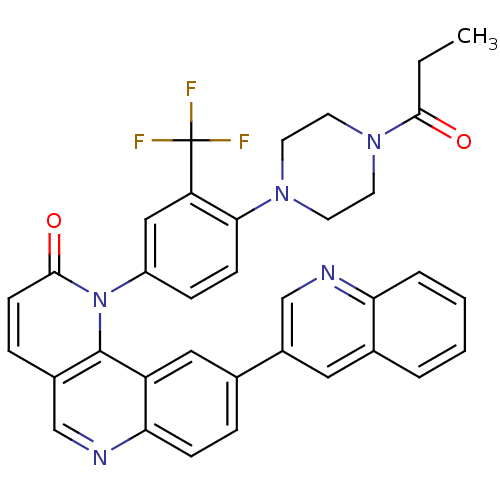
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, Q; Chang, JW; Wang, J; Kang, SA; Thoreen, CC; Markhard, A; Hur, W; Zhang, J; Sim, T; Sabatini, DM; Gray, NS Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem53:7146-55 (2010) [PubMed] Article
Liu, Q; Chang, JW; Wang, J; Kang, SA; Thoreen, CC; Markhard, A; Hur, W; Zhang, J; Sim, T; Sabatini, DM; Gray, NS Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem53:7146-55 (2010) [PubMed] Article