

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Serine/threonine-protein kinase mTOR | ||
| Ligand | BDBM50328601 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_665716 (CHEMBL1261685) | ||
| IC50 | 200±n/a nM | ||
| Citation |  Liu, Q; Chang, JW; Wang, J; Kang, SA; Thoreen, CC; Markhard, A; Hur, W; Zhang, J; Sim, T; Sabatini, DM; Gray, NS Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem53:7146-55 (2010) [PubMed] Article Liu, Q; Chang, JW; Wang, J; Kang, SA; Thoreen, CC; Markhard, A; Hur, W; Zhang, J; Sim, T; Sabatini, DM; Gray, NS Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem53:7146-55 (2010) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Serine/threonine-protein kinase mTOR | |||
| Name: | Serine/threonine-protein kinase mTOR | ||
| Synonyms: | FK506-binding protein 12-rapamycin complex-associated protein 1 | FKBP12-rapamycin complex-associated protein | Frap | Frap1 | MTOR_MOUSE | Mammalian target of rapamycin | Mechanistic target of rapamycin | Mtor | RAPT1 | Rapamycin target protein 1 | Serine/threonine-protein kinase mTOR | Serine/threonine-protein kinase mTOR (mTOR) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 288814.44 | ||
| Organism: | Mus musculus (Mouse) | ||
| Description: | Q9JLN9 | ||
| Residue: | 2549 | ||
| Sequence: |
| ||
| BDBM50328601 | |||
| n/a | |||
| Name | BDBM50328601 | ||
| Synonyms: | 1-(4-(4-aminopiperidin-1-yl)-2-methoxyphenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one | CHEMBL1259120 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C33H29N5O2 | ||
| Mol. Mass. | 527.6157 | ||
| SMILES | COc1cc(ccc1-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cnc2ccccc2c1)N1CCC(N)CC1 |(15.76,-21.17,;15.76,-22.71,;14.43,-23.49,;13.09,-22.72,;11.76,-23.49,;11.76,-25.03,;13.09,-25.81,;14.43,-25.04,;15.75,-25.83,;15.77,-27.35,;17.1,-28.11,;18.42,-27.34,;18.4,-25.81,;17.07,-25.05,;17.05,-23.51,;17.11,-29.65,;15.77,-30.43,;14.43,-29.66,;13.11,-30.42,;11.78,-29.66,;11.78,-28.12,;13.11,-27.35,;14.43,-28.12,;10.44,-27.35,;10.45,-25.8,;9.11,-25.03,;7.78,-25.81,;6.44,-25.04,;5.1,-25.81,;5.11,-27.37,;6.45,-28.13,;7.78,-27.36,;9.11,-28.12,;10.43,-22.72,;9.1,-23.48,;7.76,-22.72,;7.76,-21.17,;6.43,-20.41,;9.1,-20.39,;10.43,-21.17,)| | ||
| Structure | 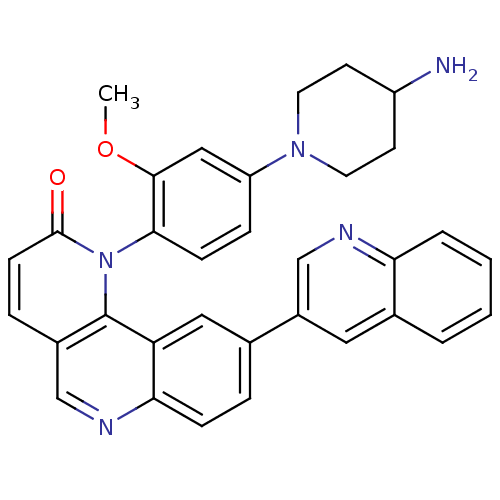
| ||