| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C motif chemokine 5 |
|---|
| Ligand | BDBM50329255 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_675123 (CHEMBL1272914) |
|---|
| IC50 | 27±n/a nM |
|---|
| Citation |  Wanner, J; Chen, L; Lemoine, RC; Kondru, R; Jekle, A; Heilek, G; deRosier, A; Ji, C; Berry, PW; Rotstein, DM Evaluation of amide replacements in CCR5 antagonists as a means to increase intrinsic permeability. Part 2: SAR optimization and pharmacokinetic profile of a homologous azacyle series. Bioorg Med Chem Lett20:6802-7 (2010) [PubMed] Article Wanner, J; Chen, L; Lemoine, RC; Kondru, R; Jekle, A; Heilek, G; deRosier, A; Ji, C; Berry, PW; Rotstein, DM Evaluation of amide replacements in CCR5 antagonists as a means to increase intrinsic permeability. Part 2: SAR optimization and pharmacokinetic profile of a homologous azacyle series. Bioorg Med Chem Lett20:6802-7 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C motif chemokine 5 |
|---|
| Name: | C-C motif chemokine 5 |
|---|
| Synonyms: | CCL5 | CCL5_HUMAN | D17S136E | EoCP | Eosinophil chemotactic cytokine | RANTES(3-68) | RANTES(4-68) | SCYA5 | SIS-delta | Small-inducible cytokine A5 | T cell-specific protein P228 | T-cell-specific protein RANTES | TCP228 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 9996.11 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1363187 |
|---|
| Residue: | 91 |
|---|
| Sequence: | MKVSAAALAVILIATALCAPASASPYSSDTTPCCFAYIARPLPRAHIKEYFYTSGKCSNP
AVVFVTRKNRQVCANPEKKWVREYINSLEMS
|
|
|
|---|
| BDBM50329255 |
|---|
| n/a |
|---|
| Name | BDBM50329255 |
|---|
| Synonyms: | 4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)piperidin-4-yl)-3-phenylpropyl)octahydropyrrolo[3,4-c]pyrrole-2-carbonyl)-2H-pyran-2-one | CHEMBL1271108 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H39N3O5S |
|---|
| Mol. Mass. | 541.702 |
|---|
| SMILES | Cc1cc(=O)oc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| |
|---|
| Structure | 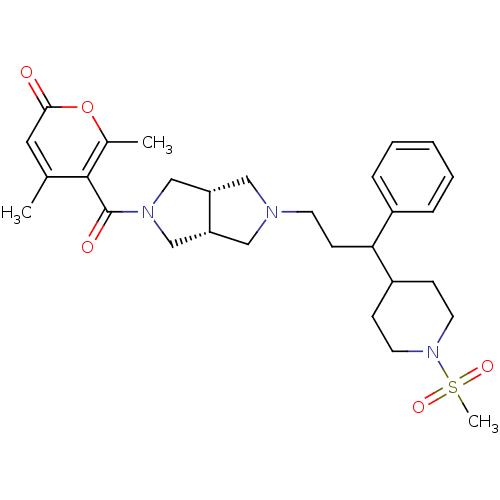
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wanner, J; Chen, L; Lemoine, RC; Kondru, R; Jekle, A; Heilek, G; deRosier, A; Ji, C; Berry, PW; Rotstein, DM Evaluation of amide replacements in CCR5 antagonists as a means to increase intrinsic permeability. Part 2: SAR optimization and pharmacokinetic profile of a homologous azacyle series. Bioorg Med Chem Lett20:6802-7 (2010) [PubMed] Article
Wanner, J; Chen, L; Lemoine, RC; Kondru, R; Jekle, A; Heilek, G; deRosier, A; Ji, C; Berry, PW; Rotstein, DM Evaluation of amide replacements in CCR5 antagonists as a means to increase intrinsic permeability. Part 2: SAR optimization and pharmacokinetic profile of a homologous azacyle series. Bioorg Med Chem Lett20:6802-7 (2010) [PubMed] Article