| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Carbonic anhydrase 13 |
|---|
| Ligand | BDBM50329826 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_675715 (CHEMBL1272349) |
|---|
| Kd | 400±n/a nM |
|---|
| Citation |  Capkauskaite, E; Baranauskiene, L; Golovenko, D; Manakova, E; Gražulis, S; Tumkevičius, S; Matulis, D Indapamide-like benzenesulfonamides as inhibitors of carbonic anhydrases I, II, VII, and XIII. Bioorg Med Chem18:7357-64 (2010) [PubMed] Article Capkauskaite, E; Baranauskiene, L; Golovenko, D; Manakova, E; Gražulis, S; Tumkevičius, S; Matulis, D Indapamide-like benzenesulfonamides as inhibitors of carbonic anhydrases I, II, VII, and XIII. Bioorg Med Chem18:7357-64 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Carbonic anhydrase 13 |
|---|
| Name: | Carbonic anhydrase 13 |
|---|
| Synonyms: | CA13 | CAH13_HUMAN | Carbonic anhydrase | Carbonic anhydrase 13 (CA XIII) | Carbonic anhydrase XIII (CA XIII) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 29445.78 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q8N1Q1 |
|---|
| Residue: | 262 |
|---|
| Sequence: | MSRLSWGYREHNGPIHWKEFFPIADGDQQSPIEIKTKEVKYDSSLRPLSIKYDPSSAKII
SNSGHSFNVDFDDTENKSVLRGGPLTGSYRLRQVHLHWGSADDHGSEHIVDGVSYAAELH
VVHWNSDKYPSFVEAAHEPDGLAVLGVFLQIGEPNSQLQKITDTLDSIKEKGKQTRFTNF
DLLSLLPPSWDYWTYPGSLTVPPLLESVTWIVLKQPINISSQQLAKFRSLLCTAEGEAAA
FLVSNHRPPQPLKGRKVRASFH
|
|
|
|---|
| BDBM50329826 |
|---|
| n/a |
|---|
| Name | BDBM50329826 |
|---|
| Synonyms: | 2-Chloro-5-[(2-isopropyl-1H-benzimidazol-1-yl)acetyl]benzenesulfonamide | CHEMBL1271477 | N-alkylated benzimidazole derivative, 4h |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H18ClN3O3S |
|---|
| Mol. Mass. | 391.872 |
|---|
| SMILES | CC(C)c1nc2ccccc2n1CC(=O)c1ccc(Cl)c(c1)S(N)(=O)=O |
|---|
| Structure | 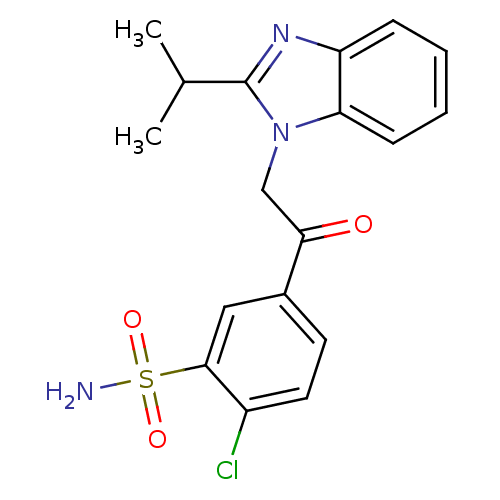
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Capkauskaite, E; Baranauskiene, L; Golovenko, D; Manakova, E; Gražulis, S; Tumkevičius, S; Matulis, D Indapamide-like benzenesulfonamides as inhibitors of carbonic anhydrases I, II, VII, and XIII. Bioorg Med Chem18:7357-64 (2010) [PubMed] Article
Capkauskaite, E; Baranauskiene, L; Golovenko, D; Manakova, E; Gražulis, S; Tumkevičius, S; Matulis, D Indapamide-like benzenesulfonamides as inhibitors of carbonic anhydrases I, II, VII, and XIII. Bioorg Med Chem18:7357-64 (2010) [PubMed] Article