| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50330022 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_676065 (CHEMBL1273059) |
|---|
| IC50 | 2±n/a nM |
|---|
| Citation |  Li, H; Xu, R; Cole, D; Clader, JW; Greenlee, WJ; Nomeir, AA; Song, L; Zhang, L Design, synthesis, and structure-activity relationship studies of N-arylsulfonyl morpholines as¿-secretase inhibitors. Bioorg Med Chem Lett20:6606-9 (2010) [PubMed] Article Li, H; Xu, R; Cole, D; Clader, JW; Greenlee, WJ; Nomeir, AA; Song, L; Zhang, L Design, synthesis, and structure-activity relationship studies of N-arylsulfonyl morpholines as¿-secretase inhibitors. Bioorg Med Chem Lett20:6606-9 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50330022 |
|---|
| n/a |
|---|
| Name | BDBM50330022 |
|---|
| Synonyms: | (3S,5R)-1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cyclopropylmethyl)morpholin-3-yl)cyclopropyl 4-(2-hydroxyethyl)-3,5-dimethylpiperazine-1-carboxylate | CHEMBL1272046 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H38ClN3O6S |
|---|
| Mol. Mass. | 556.114 |
|---|
| SMILES | C[C@H]1CN(C[C@@H](C)N1CCO)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| |
|---|
| Structure | 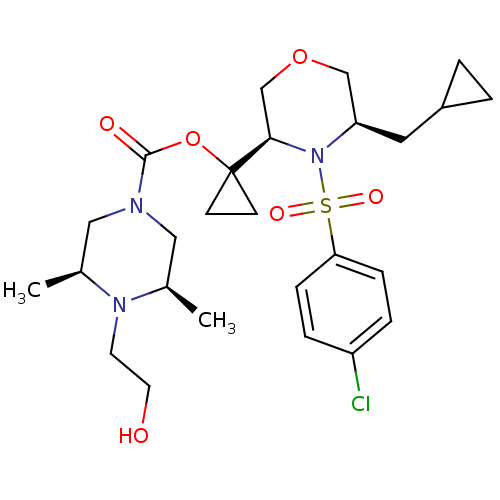
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Li, H; Xu, R; Cole, D; Clader, JW; Greenlee, WJ; Nomeir, AA; Song, L; Zhang, L Design, synthesis, and structure-activity relationship studies of N-arylsulfonyl morpholines as¿-secretase inhibitors. Bioorg Med Chem Lett20:6606-9 (2010) [PubMed] Article
Li, H; Xu, R; Cole, D; Clader, JW; Greenlee, WJ; Nomeir, AA; Song, L; Zhang, L Design, synthesis, and structure-activity relationship studies of N-arylsulfonyl morpholines as¿-secretase inhibitors. Bioorg Med Chem Lett20:6606-9 (2010) [PubMed] Article