| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Mitogen-activated protein kinase 9 |
|---|
| Ligand | BDBM50333989 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_701206 (CHEMBL1648826) |
|---|
| IC50 | 170±n/a nM |
|---|
| Citation |  Probst, GD; Bowers, S; Sealy, JM; Truong, AP; Hom, RK; Galemmo, RA; Konradi, AW; Sham, HL; Quincy, DA; Pan, H; Yao, N; Lin, M; Tóth, G; Artis, DR; Zmolek, W; Wong, K; Qin, A; Lorentzen, C; Nakamura, DF; Quinn, KP; Sauer, JM; Powell, K; Ruslim, L; Wright, S; Chereau, D; Ren, Z; Anderson, JP; Bard, F; Yednock, TA; Griswold-Prenner, I Highly selective c-Jun N-terminal kinase (JNK) 2 and 3 inhibitors with in vitro CNS-like pharmacokinetic properties prevent neurodegeneration. Bioorg Med Chem Lett21:315-9 (2010) [PubMed] Article Probst, GD; Bowers, S; Sealy, JM; Truong, AP; Hom, RK; Galemmo, RA; Konradi, AW; Sham, HL; Quincy, DA; Pan, H; Yao, N; Lin, M; Tóth, G; Artis, DR; Zmolek, W; Wong, K; Qin, A; Lorentzen, C; Nakamura, DF; Quinn, KP; Sauer, JM; Powell, K; Ruslim, L; Wright, S; Chereau, D; Ren, Z; Anderson, JP; Bard, F; Yednock, TA; Griswold-Prenner, I Highly selective c-Jun N-terminal kinase (JNK) 2 and 3 inhibitors with in vitro CNS-like pharmacokinetic properties prevent neurodegeneration. Bioorg Med Chem Lett21:315-9 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Mitogen-activated protein kinase 9 |
|---|
| Name: | Mitogen-activated protein kinase 9 |
|---|
| Synonyms: | JNK-55 | JNK2 | JNK2/JNK3 | MAPK9 | MK09_HUMAN | Mitogen-Activated Protein Kinase 9 (JNK2) | Mitogen-activated protein kinase 8/9 | PRKM9 | SAPK1A | Stress-activated protein kinase JNK2 | c-Jun N-terminal kinase 2 | c-Jun N-terminal kinase 2 (JNK2) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48131.49 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | JNK-2 was purchased from Upstate Cell Signaling Solutions (formerly Upstate Biotechnology). |
|---|
| Residue: | 424 |
|---|
| Sequence: | MSDSKCDSQFYSVQVADSTFTVLKRYQQLKPIGSGAQGIVCAAFDTVLGINVAVKKLSRP
FQNQTHAKRAYRELVLLKCVNHKNIISLLNVFTPQKTLEEFQDVYLVMELMDANLCQVIH
MELDHERMSYLLYQMLCGIKHLHSAGIIHRDLKPSNIVVKSDCTLKILDFGLARTACTNF
MMTPYVVTRYYRAPEVILGMGYKENVDIWSVGCIMGELVKGCVIFQGTDHIDQWNKVIEQ
LGTPSAEFMKKLQPTVRNYVENRPKYPGIKFEELFPDWIFPSESERDKIKTSQARDLLSK
MLVIDPDKRISVDEALRHPYITVWYDPAEAEAPPPQIYDAQLEEREHAIEEWKELIYKEV
MDWEERSKNGVVKDQPSDAAVSSNATPSQSSSINDISSMSTEQTLASDTDSSLDASTGPL
EGCR
|
|
|
|---|
| BDBM50333989 |
|---|
| n/a |
|---|
| Name | BDBM50333989 |
|---|
| Synonyms: | 4-(naphthalen-2-yl)-3-(2-(tetrahydro-2H-pyran-4-ylamino)pyridin-4-yl)-1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)-1H-1,2,4-triazol-5(4H)-one | CHEMBL1644645 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H31F3N6O2 |
|---|
| Mol. Mass. | 552.5906 |
|---|
| SMILES | FC(F)(F)CN1CCC(CC1)n1nc(-c2ccnc(NC3CCOCC3)c2)n(-c2ccc3ccccc3c2)c1=O |
|---|
| Structure | 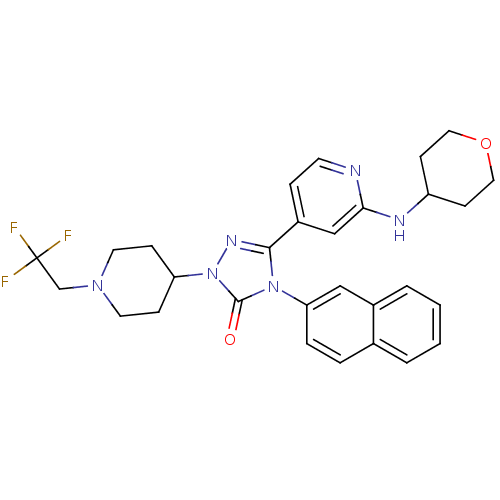
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Probst, GD; Bowers, S; Sealy, JM; Truong, AP; Hom, RK; Galemmo, RA; Konradi, AW; Sham, HL; Quincy, DA; Pan, H; Yao, N; Lin, M; Tóth, G; Artis, DR; Zmolek, W; Wong, K; Qin, A; Lorentzen, C; Nakamura, DF; Quinn, KP; Sauer, JM; Powell, K; Ruslim, L; Wright, S; Chereau, D; Ren, Z; Anderson, JP; Bard, F; Yednock, TA; Griswold-Prenner, I Highly selective c-Jun N-terminal kinase (JNK) 2 and 3 inhibitors with in vitro CNS-like pharmacokinetic properties prevent neurodegeneration. Bioorg Med Chem Lett21:315-9 (2010) [PubMed] Article
Probst, GD; Bowers, S; Sealy, JM; Truong, AP; Hom, RK; Galemmo, RA; Konradi, AW; Sham, HL; Quincy, DA; Pan, H; Yao, N; Lin, M; Tóth, G; Artis, DR; Zmolek, W; Wong, K; Qin, A; Lorentzen, C; Nakamura, DF; Quinn, KP; Sauer, JM; Powell, K; Ruslim, L; Wright, S; Chereau, D; Ren, Z; Anderson, JP; Bard, F; Yednock, TA; Griswold-Prenner, I Highly selective c-Jun N-terminal kinase (JNK) 2 and 3 inhibitors with in vitro CNS-like pharmacokinetic properties prevent neurodegeneration. Bioorg Med Chem Lett21:315-9 (2010) [PubMed] Article