| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2C9 |
|---|
| Ligand | BDBM50336378 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_716791 (CHEMBL1670713) |
|---|
| IC50 | 7100±n/a nM |
|---|
| Citation |  Richter, HG; Benson, GM; Bleicher, KH; Blum, D; Chaput, E; Clemann, N; Feng, S; Gardes, C; Grether, U; Hartman, P; Kuhn, B; Martin, RE; Plancher, JM; Rudolph, MG; Schuler, F; Taylor, S Optimization of a novel class of benzimidazole-based farnesoid X receptor (FXR) agonists to improve physicochemical and ADME properties. Bioorg Med Chem Lett21:1134-40 (2011) [PubMed] Article Richter, HG; Benson, GM; Bleicher, KH; Blum, D; Chaput, E; Clemann, N; Feng, S; Gardes, C; Grether, U; Hartman, P; Kuhn, B; Martin, RE; Plancher, JM; Rudolph, MG; Schuler, F; Taylor, S Optimization of a novel class of benzimidazole-based farnesoid X receptor (FXR) agonists to improve physicochemical and ADME properties. Bioorg Med Chem Lett21:1134-40 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2C9 |
|---|
| Name: | Cytochrome P450 2C9 |
|---|
| Synonyms: | (R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55636.33 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11712 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKV
YGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKW
KEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICS
IIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFM
KSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTE
TTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYID
LLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFK
KSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVP
PFYQLCFIPV
|
|
|
|---|
| BDBM50336378 |
|---|
| n/a |
|---|
| Name | BDBM50336378 |
|---|
| Synonyms: | (S)-2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo[d]imidazol-1-yl)-2-cyclohexyl-N-(2-fluoro-4-(1H-tetrazol-5-yl)phenyl)acetamide | CHEMBL1668257 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H23ClF3N7O |
|---|
| Mol. Mass. | 565.977 |
|---|
| SMILES | Fc1cc2nc(-c3ccc(Cl)cc3)n([C@@H](C3CCCCC3)C(=O)Nc3ccc(cc3F)-c3nnn[nH]3)c2cc1F |r| |
|---|
| Structure | 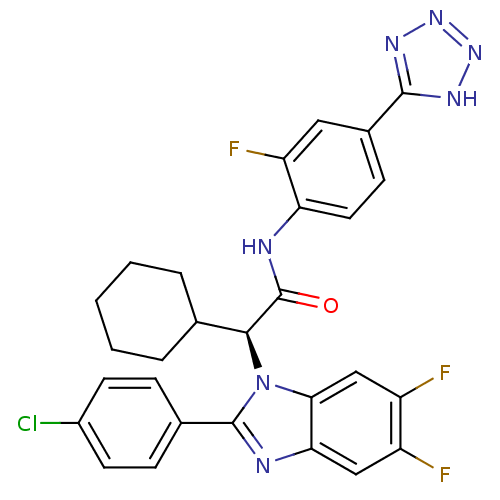
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Richter, HG; Benson, GM; Bleicher, KH; Blum, D; Chaput, E; Clemann, N; Feng, S; Gardes, C; Grether, U; Hartman, P; Kuhn, B; Martin, RE; Plancher, JM; Rudolph, MG; Schuler, F; Taylor, S Optimization of a novel class of benzimidazole-based farnesoid X receptor (FXR) agonists to improve physicochemical and ADME properties. Bioorg Med Chem Lett21:1134-40 (2011) [PubMed] Article
Richter, HG; Benson, GM; Bleicher, KH; Blum, D; Chaput, E; Clemann, N; Feng, S; Gardes, C; Grether, U; Hartman, P; Kuhn, B; Martin, RE; Plancher, JM; Rudolph, MG; Schuler, F; Taylor, S Optimization of a novel class of benzimidazole-based farnesoid X receptor (FXR) agonists to improve physicochemical and ADME properties. Bioorg Med Chem Lett21:1134-40 (2011) [PubMed] Article