

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Dihydrofolate reductase | ||
| Ligand | BDBM50298821 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_723694 (CHEMBL1677364) | ||
| IC50 | 61±n/a nM | ||
| Citation |  Bourne, CR; Barrow, EW; Bunce, RA; Bourne, PC; Berlin, KD; Barrow, WW Inhibition of antibiotic-resistant Staphylococcus aureus by the broad-spectrum dihydrofolate reductase inhibitor RAB1. Antimicrob Agents Chemother54:3825-33 (2010) [PubMed] Article Bourne, CR; Barrow, EW; Bunce, RA; Bourne, PC; Berlin, KD; Barrow, WW Inhibition of antibiotic-resistant Staphylococcus aureus by the broad-spectrum dihydrofolate reductase inhibitor RAB1. Antimicrob Agents Chemother54:3825-33 (2010) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Dihydrofolate reductase | |||
| Name: | Dihydrofolate reductase | ||
| Synonyms: | DYR_STAAU | Dihydrofolate Reductase (DHFR) | Dihydrofolate reductase | Dihydrofolate reductase (DfrB) | Tetrahydrofolate dehydrogenase | folA | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 18249.71 | ||
| Organism: | Staphylococcus aureus | ||
| Description: | n/a | ||
| Residue: | 159 | ||
| Sequence: |
| ||
| BDBM50298821 | |||
| n/a | |||
| Name | BDBM50298821 | ||
| Synonyms: | (S)-5-(3-(2-methoxy-2',6'-dimethylbiphenyl-4-yl)but-1-ynyl)-6-methylpyrimidine-2,4-diamine | CHEMBL577790 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C24H26N4O | ||
| Mol. Mass. | 386.4894 | ||
| SMILES | COc1cc(ccc1-c1c(C)cccc1C)[C@H](C)C#Cc1c(C)nc(N)nc1N |r,wU:16.18,(1.34,-3.46,;2.67,-2.68,;2.66,-1.15,;1.33,-.38,;1.32,1.16,;2.64,1.94,;3.98,1.18,;4,-.37,;5.33,-1.13,;6.65,-.35,;6.63,1.19,;7.99,-1.1,;8,-2.65,;6.67,-3.43,;5.33,-2.67,;4,-3.44,;-.01,1.92,;-.02,3.46,;-1.34,1.14,;-2.67,.36,;-4,-.41,;-4,-1.96,;-2.66,-2.73,;-5.33,-2.73,;-6.67,-1.96,;-8,-2.73,;-6.67,-.42,;-5.34,.35,;-5.34,1.89,)| | ||
| Structure | 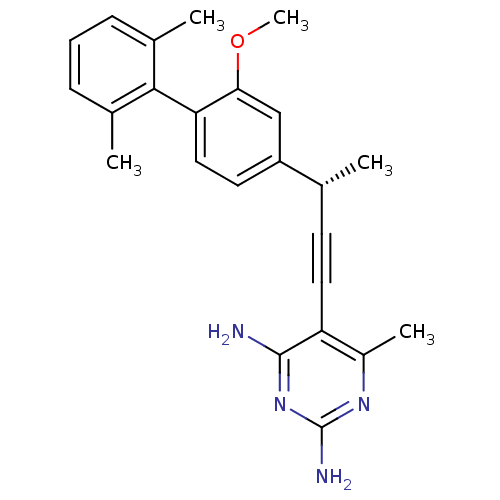
| ||