| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, inducible |
|---|
| Ligand | BDBM50124535 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_740009 (CHEMBL1763069) |
|---|
| IC50 | 41±n/a nM |
|---|
| Citation |  Cheshire, DR; Åberg, A; Andersson, GM; Andrews, G; Beaton, HG; Birkinshaw, TN; Boughton-Smith, N; Connolly, S; Cook, TR; Cooper, A; Cooper, SL; Cox, D; Dixon, J; Gensmantel, N; Hamley, PJ; Harrison, R; Hartopp, P; Käck, H; Leeson, PD; Luker, T; Mete, A; Millichip, I; Nicholls, DJ; Pimm, AD; St-Gallay, SA; Wallace, AV The discovery of novel, potent and highly selective inhibitors of inducible nitric oxide synthase (iNOS). Bioorg Med Chem Lett21:2468-71 (2011) [PubMed] Article Cheshire, DR; Åberg, A; Andersson, GM; Andrews, G; Beaton, HG; Birkinshaw, TN; Boughton-Smith, N; Connolly, S; Cook, TR; Cooper, A; Cooper, SL; Cox, D; Dixon, J; Gensmantel, N; Hamley, PJ; Harrison, R; Hartopp, P; Käck, H; Leeson, PD; Luker, T; Mete, A; Millichip, I; Nicholls, DJ; Pimm, AD; St-Gallay, SA; Wallace, AV The discovery of novel, potent and highly selective inhibitors of inducible nitric oxide synthase (iNOS). Bioorg Med Chem Lett21:2468-71 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, inducible |
|---|
| Name: | Nitric oxide synthase, inducible |
|---|
| Synonyms: | HEP-NOS | Hepatocyte NOS | Inducible NO synthase | Inducible NOS | NOS type II | NOS2 | NOS2A | NOS2_HUMAN | Nitric oxide synthase, inducible (iNOS) | iNOS |
|---|
| Type: | Homodimer |
|---|
| Mol. Mass.: | 131141.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35228 |
|---|
| Residue: | 1153 |
|---|
| Sequence: | MACPWKFLFKTKFHQYAMNGEKDINNNVEKAPCATSSPVTQDDLQYHNLSKQQNESPQPL
VETGKKSPESLVKLDATPLSSPRHVRIKNWGSGMTFQDTLHHKAKGILTCRSKSCLGSIM
TPKSLTRGPRDKPTPPDELLPQAIEFVNQYYGSFKEAKIEEHLARVEAVTKEIETTGTYQ
LTGDELIFATKQAWRNAPRCIGRIQWSNLQVFDARSCSTAREMFEHICRHVRYSTNNGNI
RSAITVFPQRSDGKHDFRVWNAQLIRYAGYQMPDGSIRGDPANVEFTQLCIDLGWKPKYG
RFDVVPLVLQANGRDPELFEIPPDLVLEVAMEHPKYEWFRELELKWYALPAVANMLLEVG
GLEFPGCPFNGWYMGTEIGVRDFCDVQRYNILEEVGRRMGLETHKLASLWKDQAVVEINI
AVLHSFQKQNVTIMDHHSAAESFMKYMQNEYRSRGGCPADWIWLVPPMSGSITPVFHQEM
LNYVLSPFYYYQVEAWKTHVWQDEKRRPKRREIPLKVLVKAVLFACMLMRKTMASRVRVT
ILFATETGKSEALAWDLGALFSCAFNPKVVCMDKYRLSCLEEERLLLVVTSTFGNGDCPG
NGEKLKKSLFMLKELNNKFRYAVFGLGSSMYPRFCAFAHDIDQKLSHLGASQLTPMGEGD
ELSGQEDAFRSWAVQTFKAACETFDVRGKQHIQIPKLYTSNVTWDPHHYRLVQDSQPLDL
SKALSSMHAKNVFTMRLKSRQNLQSPTSSRATILVELSCEDGQGLNYLPGEHLGVCPGNQ
PALVQGILERVVDGPTPHQTVRLEALDESGSYWVSDKRLPPCSLSQALTYFLDITTPPTQ
LLLQKLAQVATEEPERQRLEALCQPSEYSKWKFTNSPTFLEVLEEFPSLRVSAGFLLSQL
PILKPRFYSISSSRDHTPTEIHLTVAVVTYHTRDGQGPLHHGVCSTWLNSLKPQDPVPCF
VRNASGFHLPEDPSHPCILIGPGTGIAPFRSFWQQRLHDSQHKGVRGGRMTLVFGCRRPD
EDHIYQEEMLEMAQKGVLHAVHTAYSRLPGKPKVYVQDILRQQLASEVLRVLHKEPGHLY
VCGDVRMARDVAHTLKQLVAAKLKLNEEQVEDYFFQLKSQKRYHEDIFGAVFPYEAKKDR
VAVQPSSLEMSAL
|
|
|
|---|
| BDBM50124535 |
|---|
| n/a |
|---|
| Name | BDBM50124535 |
|---|
| Synonyms: | 1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[PIPERIDINE-4,2'(1'H)-QUINAZOLINE]-4'-AMINE | 5-(4'-amino-5',8'-difluoro-1'H-spiro[piperidine-4,2'-quinazoline]-1-ylcarbonyl)picolinonitrile | 5-(4-Amino-5,8-difluorospiro[1H-quinazoline-2,4'-piperidine]-1'-carbonyl)pyridine-2-carbonitrile, 3 | 5-[(4'-amino-5',8'-difluoro-1H,1'H-spiro[piperidine-4,2'-quinazolin]-1-yl)carbonyl]pyridine-2-carbonitrile | 5-[4'-amino-5',8'-difluorospiro[hexahydropyridine-4,2'-(1',2'-dihydroquinazoline)]-1-ylcarbonyl]-2-pyridinecarbonitrile | AR-C102222 | CHEMBL447183 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H16F2N6O |
|---|
| Mol. Mass. | 382.3667 |
|---|
| SMILES | NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| |
|---|
| Structure | 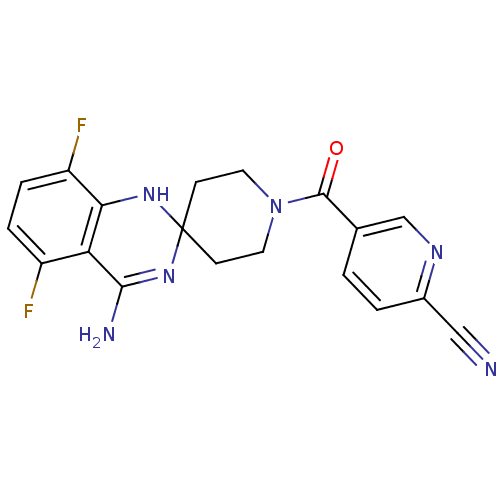
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cheshire, DR; Åberg, A; Andersson, GM; Andrews, G; Beaton, HG; Birkinshaw, TN; Boughton-Smith, N; Connolly, S; Cook, TR; Cooper, A; Cooper, SL; Cox, D; Dixon, J; Gensmantel, N; Hamley, PJ; Harrison, R; Hartopp, P; Käck, H; Leeson, PD; Luker, T; Mete, A; Millichip, I; Nicholls, DJ; Pimm, AD; St-Gallay, SA; Wallace, AV The discovery of novel, potent and highly selective inhibitors of inducible nitric oxide synthase (iNOS). Bioorg Med Chem Lett21:2468-71 (2011) [PubMed] Article
Cheshire, DR; Åberg, A; Andersson, GM; Andrews, G; Beaton, HG; Birkinshaw, TN; Boughton-Smith, N; Connolly, S; Cook, TR; Cooper, A; Cooper, SL; Cox, D; Dixon, J; Gensmantel, N; Hamley, PJ; Harrison, R; Hartopp, P; Käck, H; Leeson, PD; Luker, T; Mete, A; Millichip, I; Nicholls, DJ; Pimm, AD; St-Gallay, SA; Wallace, AV The discovery of novel, potent and highly selective inhibitors of inducible nitric oxide synthase (iNOS). Bioorg Med Chem Lett21:2468-71 (2011) [PubMed] Article