| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histamine H1 receptor |
|---|
| Ligand | BDBM50341447 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_741667 (CHEMBL1769659) |
|---|
| Kd | 0.794±n/a nM |
|---|
| Citation |  Procopiou, PA; Browning, C; Buckley, JM; Clark, KL; Fechner, L; Gore, PM; Hancock, AP; Hodgson, ST; Holmes, DS; Kranz, M; Looker, BE; Morriss, KM; Parton, DL; Russell, LJ; Slack, RJ; Sollis, SL; Vile, S; Watts, CJ The discovery of phthalazinone-based human H1 and H3 single-ligand antagonists suitable for intranasal administration for the treatment of allergic rhinitis. J Med Chem54:2183-95 (2011) [PubMed] Article Procopiou, PA; Browning, C; Buckley, JM; Clark, KL; Fechner, L; Gore, PM; Hancock, AP; Hodgson, ST; Holmes, DS; Kranz, M; Looker, BE; Morriss, KM; Parton, DL; Russell, LJ; Slack, RJ; Sollis, SL; Vile, S; Watts, CJ The discovery of phthalazinone-based human H1 and H3 single-ligand antagonists suitable for intranasal administration for the treatment of allergic rhinitis. J Med Chem54:2183-95 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histamine H1 receptor |
|---|
| Name: | Histamine H1 receptor |
|---|
| Synonyms: | H1R | HH1R | HISTAMINE H1 | HRH1 | HRH1_HUMAN |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 55808.72 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Cell pellets from SK-N-MC cells transfected with human H1 receptor were used in binding assay. |
|---|
| Residue: | 487 |
|---|
| Sequence: | MSLPNSSCLLEDKMCEGNKTTMASPQLMPLVVVLSTICLVTVGLNLLVLYAVRSERKLHT
VGNLYIVSLSVADLIVGAVVMPMNILYLLMSKWSLGRPLCLFWLSMDYVASTASIFSVFI
LCIDRYRSVQQPLRYLKYRTKTRASATILGAWFLSFLWVIPILGWNHFMQQTSVRREDKC
ETDFYDVTWFKVMTAIINFYLPTLLMLWFYAKIYKAVRQHCQHRELINRSLPSFSEIKLR
PENPKGDAKKPGKESPWEVLKRKPKDAGGGSVLKSPSQTPKEMKSPVVFSQEDDREVDKL
YCFPLDIVHMQAAAEGSSRDYVAVNRSHGQLKTDEQGLNTHGASEISEDQMLGDSQSFSR
TDSDTTTETAPGKGKLRSGSNTGLDYIKFTWKRLRSHSRQYVSGLHMNRERKAAKQLGFI
MAAFILCWIPYFIFFMVIAFCKNCCNEHLHMFTIWLGYINSTLNPLIYPLCNENFKKTFK
RILHIRS
|
|
|
|---|
| BDBM50341447 |
|---|
| n/a |
|---|
| Name | BDBM50341447 |
|---|
| Synonyms: | 4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(hexahydro-1H-azepin-1-yl)propyl]oxy}phenyl)butyl]-2-pyrrolidinyl}methyl)-1(2H)-phthalazinone | CHEMBL1767164 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C39H49ClN4O2 |
|---|
| Mol. Mass. | 641.285 |
|---|
| SMILES | Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| |
|---|
| Structure | 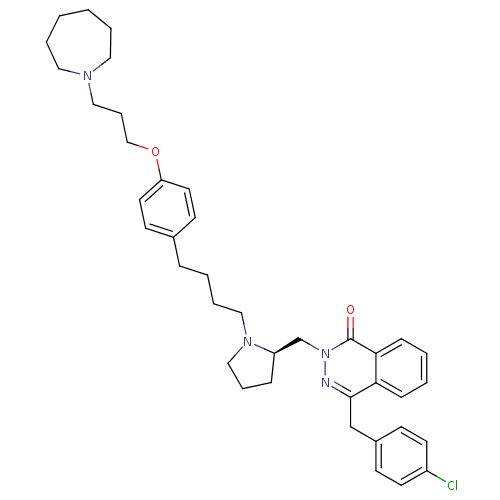
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Procopiou, PA; Browning, C; Buckley, JM; Clark, KL; Fechner, L; Gore, PM; Hancock, AP; Hodgson, ST; Holmes, DS; Kranz, M; Looker, BE; Morriss, KM; Parton, DL; Russell, LJ; Slack, RJ; Sollis, SL; Vile, S; Watts, CJ The discovery of phthalazinone-based human H1 and H3 single-ligand antagonists suitable for intranasal administration for the treatment of allergic rhinitis. J Med Chem54:2183-95 (2011) [PubMed] Article
Procopiou, PA; Browning, C; Buckley, JM; Clark, KL; Fechner, L; Gore, PM; Hancock, AP; Hodgson, ST; Holmes, DS; Kranz, M; Looker, BE; Morriss, KM; Parton, DL; Russell, LJ; Slack, RJ; Sollis, SL; Vile, S; Watts, CJ The discovery of phthalazinone-based human H1 and H3 single-ligand antagonists suitable for intranasal administration for the treatment of allergic rhinitis. J Med Chem54:2183-95 (2011) [PubMed] Article