| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50342765 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_744405 (CHEMBL1772359) |
|---|
| IC50 | 22±n/a nM |
|---|
| Citation |  Li, YP; Ning, FX; Yang, MB; Li, YC; Nie, MH; Ou, TM; Tan, JH; Huang, SL; Li, D; Gu, LQ; Huang, ZS Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur J Med Chem46:1572-81 (2011) [PubMed] Article Li, YP; Ning, FX; Yang, MB; Li, YC; Nie, MH; Ou, TM; Tan, JH; Huang, SL; Li, D; Gu, LQ; Huang, ZS Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur J Med Chem46:1572-81 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_ELEEL | Acetylcholinesterase (AChE) | Acetylcholinesterase (EeAChE) | ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 71812.79 |
|---|
| Organism: | Electrophorus electricus (Electric eel) |
|---|
| Description: | n/a |
|---|
| Residue: | 633 |
|---|
| Sequence: | MKILDALLFPVIFIMFFIHLSIAQTDPELTIMTRLGQVQGTRLPVPDRSHVIAFLGIPFA
EPPLGKMRFKPPEPKKPWNDVFDARDYPSACYQYVDTSYPGFSGTEMWNPNRMMSEDCLY
LNVWVPATPRPHNLTVMVWIYGGGFYSGSSSLDVYDGRYLAHSEKVVVVSMNYRVSAFGF
LALNGSAEAPGNVGLLDQRLALQWVQDNIHFFGGNPKQVTIFGESAGAASVGMHLLSPDS
RPKFTRAILQSGVPNGPWRTVSFDEARRRAIKLGRLVGCPDGNDTDLIDCLRSKQPQDLI
DQEWLVLPFSGLFRFSFVPVIDGVVFPDTPEAMLNSGNFKDTQILLGVNQNEGSYFLIYG
APGFSKDNESLITREDFLQGVKMSVPHANEIGLEAVILQYTDWMDEDNPIKNREAMDDIV
GDHNVVCPLQHFAKMYAQYSILQGQTGTASQGNLGWGNSGSASNSGNSQVSVYLYMFDHR
ASNLVWPEWMGVIHGYEIEFVFGLPLEKRLNYTLEEEKLSRRMMKYWANFARTGNPNINV
DGSIDSRRRWPVFTSTEQKHVGLNTDSLKVHKGLKSQFCALWNRFLPRLLNVTENIDDAE
RQWKAEFHRWSSYMMHWKNQFDHYSKQERCTNL
|
|
|
|---|
| BDBM50342765 |
|---|
| n/a |
|---|
| Name | BDBM50342765 |
|---|
| Synonyms: | 4-(3-(Piperidin-1-yl)propoxy)-7H-dibenzo[de,h]quinolin-7-one | CHEMBL1770206 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H24N2O2 |
|---|
| Mol. Mass. | 372.4596 |
|---|
| SMILES | O=C1c2ccccc2-c2nccc3c(OCCCN4CCCCC4)ccc1c23 |
|---|
| Structure | 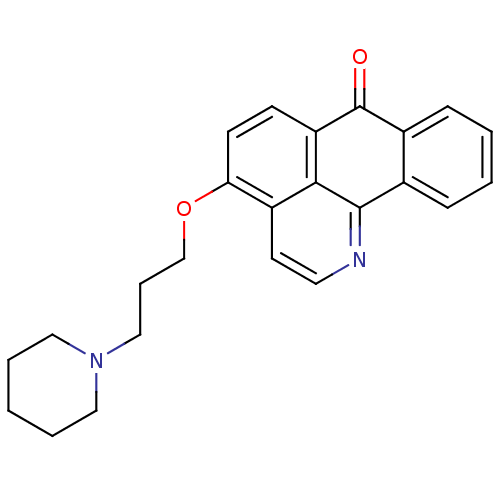
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Li, YP; Ning, FX; Yang, MB; Li, YC; Nie, MH; Ou, TM; Tan, JH; Huang, SL; Li, D; Gu, LQ; Huang, ZS Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur J Med Chem46:1572-81 (2011) [PubMed] Article
Li, YP; Ning, FX; Yang, MB; Li, YC; Nie, MH; Ou, TM; Tan, JH; Huang, SL; Li, D; Gu, LQ; Huang, ZS Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur J Med Chem46:1572-81 (2011) [PubMed] Article