| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Ligand | BDBM50342783 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_744419 (CHEMBL1772373) |
|---|
| IC50 | 420000±n/a nM |
|---|
| Citation |  Mravljak, J; Monasson, O; Al-Dabbagh, B; Crouvoisier, M; Bouhss, A; Gravier-Pelletier, C; Le Merrer, Y Synthesis and biological evaluation of a diazepanone-based library of liposidomycins analogs as MraY inhibitors. Eur J Med Chem46:1582-92 (2011) [PubMed] Article Mravljak, J; Monasson, O; Al-Dabbagh, B; Crouvoisier, M; Bouhss, A; Gravier-Pelletier, C; Le Merrer, Y Synthesis and biological evaluation of a diazepanone-based library of liposidomycins analogs as MraY inhibitors. Eur J Med Chem46:1582-92 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Name: | Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Synonyms: | MRAY_STAAU | mraY |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 35238.55 |
|---|
| Organism: | Staphylococcus aureus (strain MRSA252) |
|---|
| Description: | ChEMBL_827497 |
|---|
| Residue: | 321 |
|---|
| Sequence: | MIFVYALLALVITFVLVPVLIPTLKRMKFGQSIREEGPQSHMKKTGTPTMGGLTFLLSIV
ITSLVAIIFVDQANPIILLLFVTIGFGLIGFIDDYIIVVKKNNQGLTSKQKFLAQIGIAI
IFFVLSNVFHLVNFSTSIHIPFTNVAIPLSFAYVIFIVFWQVGFSNAVNLTDGLDGLATG
LSIIGFTMYAIMSFVLGETAIGIFCIIMLFALLGFLPYNINPAKVFMGDTGSLALGGIFA
TISIMLNQELSLIFIGLVFVIETLSVMLQVASFKLTGKRIFKMSPIHHHFELIGWSEWKV
VTVFWAVGLISGLIGLWIGVH
|
|
|
|---|
| BDBM50342783 |
|---|
| n/a |
|---|
| Name | BDBM50342783 |
|---|
| Synonyms: | (3S,6S,7R)-3,7-Di-(5-azido-5-deoxy-2,3-O-isopentylidene-beta-D-ribos-1-yl-methyl)-4-N-(5''-(uracil-1'-yl)pentyl)-6-palmitoyloxy-1,4-diazepan-2-one | CHEMBL1770418 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C52H86N10O13 |
|---|
| Mol. Mass. | 1059.2984 |
|---|
| SMILES | CCCCCCCCCCCCCCCC(=O)O[C@H]1CN(CCCCCn2ccc(=O)[nH]c2=O)[C@@H](CO[C@@H]2O[C@H](CN=[N+]=[N-])[C@H]3OC(CC)(CC)O[C@@H]23)C(=O)N[C@@H]1CO[C@@H]1O[C@H](CN=[N+]=[N-])[C@H]2OC(CC)(CC)O[C@@H]12 |r| |
|---|
| Structure | 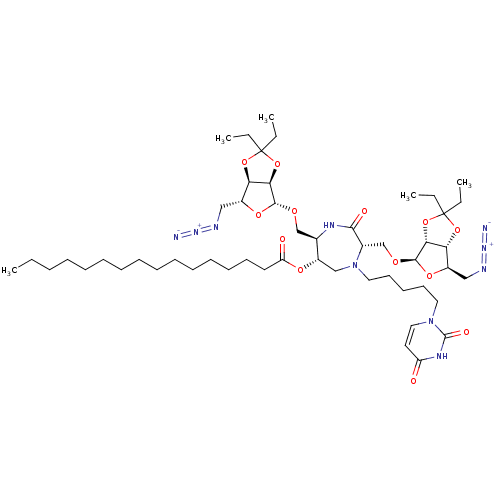
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Mravljak, J; Monasson, O; Al-Dabbagh, B; Crouvoisier, M; Bouhss, A; Gravier-Pelletier, C; Le Merrer, Y Synthesis and biological evaluation of a diazepanone-based library of liposidomycins analogs as MraY inhibitors. Eur J Med Chem46:1582-92 (2011) [PubMed] Article
Mravljak, J; Monasson, O; Al-Dabbagh, B; Crouvoisier, M; Bouhss, A; Gravier-Pelletier, C; Le Merrer, Y Synthesis and biological evaluation of a diazepanone-based library of liposidomycins analogs as MraY inhibitors. Eur J Med Chem46:1582-92 (2011) [PubMed] Article