| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, brain |
|---|
| Ligand | BDBM50352520 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_767162 (CHEMBL1826313) |
|---|
| Ki | 26±n/a nM |
|---|
| Citation |  Xue, F; Kraus, JM; Labby, KJ; Ji, H; Mataka, J; Xia, G; Li, H; Delker, SL; Roman, LJ; Martásek, P; Poulos, TL; Silverman, RB Improved synthesis of chiral pyrrolidine inhibitors and their binding properties to neuronal nitric oxide synthase. J Med Chem54:6399-403 (2011) [PubMed] Article Xue, F; Kraus, JM; Labby, KJ; Ji, H; Mataka, J; Xia, G; Li, H; Delker, SL; Roman, LJ; Martásek, P; Poulos, TL; Silverman, RB Improved synthesis of chiral pyrrolidine inhibitors and their binding properties to neuronal nitric oxide synthase. J Med Chem54:6399-403 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, brain |
|---|
| Name: | Nitric oxide synthase, brain |
|---|
| Synonyms: | Bnos | N-NOS | NC-NOS | NOS | NOS type I nNOS | NOS1_RAT | Neuronal nitric oxide synthase | Neuronal nitric oxide synthase (nNOS) | Nitric Oxide Synthase, brain | Nitric oxide synthase (nNOS) | Nitric oxide synthase, brain (nNOS) | Nitric-oxide synthase, brain | Nitric-oxide synthase, brain (nNOS) | Nitrogen oxide synthase - neuronal | Nos1 | Peptidyl-cysteine S-nitrosylase NOS1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 160570.98 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Recombinant nNOS overexpressed in E. coli was used in enzyme assays. |
|---|
| Residue: | 1429 |
|---|
| Sequence: | MEENTFGVQQIQPNVISVRLFKRKVGGLGFLVKERVSKPPVIISDLIRGGAAEQSGLIQA
GDIILAVNDRPLVDLSYDSALEVLRGIASETHVVLILRGPEGFTTHLETTFTGDGTPKTI
RVTQPLGPPTKAVDLSHQPSASKDQSLAVDRVTGLGNGPQHAQGHGQGAGSVSQANGVAI
DPTMKSTKANLQDIGEHDELLKEIEPVLSILNSGSKATNRGGPAKAEMKDTGIQVDRDLD
GKSHKAPPLGGDNDRVFNDLWGKDNVPVILNNPYSEKEQSPTSGKQSPTKNGSPSRCPRF
LKVKNWETDVVLTDTLHLKSTLETGCTEHICMGSIMLPSQHTRKPEDVRTKDQLFPLAKE
FLDQYYSSIKRFGSKAHMDRLEEVNKEIESTSTYQLKDTELIYGAKHAWRNASRCVGRIQ
WSKLQVFDARDCTTAHGMFNYICNHVKYATNKGNLRSAITIFPQRTDGKHDFRVWNSQLI
RYAGYKQPDGSTLGDPANVQFTEICIQQGWKAPRGRFDVLPLLLQANGNDPELFQIPPEL
VLEVPIRHPKFDWFKDLGLKWYGLPAVSNMLLEIGGLEFSACPFSGWYMGTEIGVRDYCD
NSRYNILEEVAKKMDLDMRKTSSLWKDQALVEINIAVLYSFQSDKVTIVDHHSATESFIK
HMENEYRCRGGCPADWVWIVPPMSGSITPVFHQEMLNYRLTPSFEYQPDPWNTHVWKGTN
GTPTKRRAIGFKKLAEAVKFSAKLMGQAMAKRVKATILYATETGKSQAYAKTLCEIFKHA
FDAKAMSMEEYDIVHLEHEALVLVVTSTFGNGDPPENGEKFGCALMEMRHPNSVQEERKS
YKVRFNSVSSYSDSRKSSGDGPDLRDNFESTGPLANVRFSVFGLGSRAYPHFCAFGHAVD
TLLEELGGERILKMREGDELCGQEEAFRTWAKKVFKAACDVFCVGDDVNIEKPNNSLISN
DRSWKRNKFRLTYVAEAPDLTQGLSNVHKKRVSAARLLSRQNLQSPKFSRSTIFVRLHTN
GNQELQYQPGDHLGVFPGNHEDLVNALIERLEDAPPANHVVKVEMLEERNTALGVISNWK
DESRLPPCTIFQAFKYYLDITTPPTPLQLQQFASLATNEKEKQRLLVLSKGLQEYEEWKW
GKNPTMVEVLEEFPSIQMPATLLLTQLSLLQPRYYSISSSPDMYPDEVHLTVAIVSYHTR
DGEGPVHHGVCSSWLNRIQADDVVPCFVRGAPSFHLPRNPQVPCILVGPGTGIAPFRSFW
QQRQFDIQHKGMNPCPMVLVFGCRQSKIDHIYREETLQAKNKGVFRELYTAYSREPDRPK
KYVQDVLQEQLAESVYRALKEQGGHIYVCGDVTMAADVLKAIQRIMTQQGKLSEEDAGVF
ISRLRDDNRYHEDIFGVTLRTYEVTNRLRSESIAFIEESKKDADEVFSS
|
|
|
|---|
| BDBM50352520 |
|---|
| n/a |
|---|
| Name | BDBM50352520 |
|---|
| Synonyms: | CHEMBL1824857 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H28F2N4O |
|---|
| Mol. Mass. | 390.47 |
|---|
| SMILES | Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCC(F)c2cccc(F)c2)c1 |r| |
|---|
| Structure | 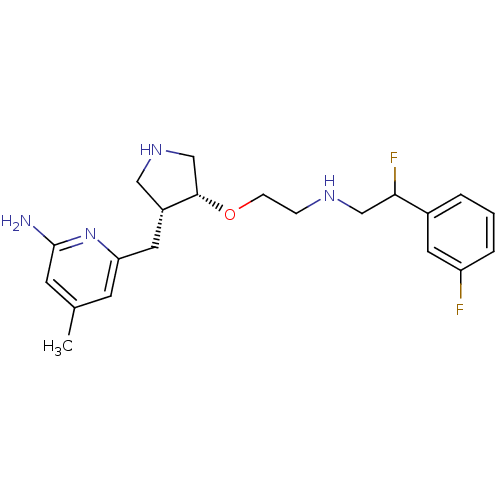
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xue, F; Kraus, JM; Labby, KJ; Ji, H; Mataka, J; Xia, G; Li, H; Delker, SL; Roman, LJ; Martásek, P; Poulos, TL; Silverman, RB Improved synthesis of chiral pyrrolidine inhibitors and their binding properties to neuronal nitric oxide synthase. J Med Chem54:6399-403 (2011) [PubMed] Article
Xue, F; Kraus, JM; Labby, KJ; Ji, H; Mataka, J; Xia, G; Li, H; Delker, SL; Roman, LJ; Martásek, P; Poulos, TL; Silverman, RB Improved synthesis of chiral pyrrolidine inhibitors and their binding properties to neuronal nitric oxide synthase. J Med Chem54:6399-403 (2011) [PubMed] Article