| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50342133 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_769540 (CHEMBL1833287) |
|---|
| IC50 | 8300±n/a nM |
|---|
| Citation |  Gomaa, MS; Bridgens, CE; Veal, GJ; Redfern, CP; Brancale, A; Armstrong, JL; Simons, C Synthesis and biological evaluation of 3-(1H-imidazol- and triazol-1-yl)-2,2-dimethyl-3-[4-(naphthalen-2-ylamino)phenyl]propyl derivatives as small molecule inhibitors of retinoic acid 4-hydroxylase (CYP26). J Med Chem54:6803-11 (2011) [PubMed] Article Gomaa, MS; Bridgens, CE; Veal, GJ; Redfern, CP; Brancale, A; Armstrong, JL; Simons, C Synthesis and biological evaluation of 3-(1H-imidazol- and triazol-1-yl)-2,2-dimethyl-3-[4-(naphthalen-2-ylamino)phenyl]propyl derivatives as small molecule inhibitors of retinoic acid 4-hydroxylase (CYP26). J Med Chem54:6803-11 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50342133 |
|---|
| n/a |
|---|
| Name | BDBM50342133 |
|---|
| Synonyms: | Benzothiazol-2-yl-[4-((1S,2S)-2-dimethylamino-1-imidazol-1-yl-propyl)-phenyl]-amine | CHEMBL517438 | N-(4-((1R,2R)-2-(dimethylamino)-1-(1H-imidazol-1-yl)propyl)phenyl)benzo[d]thiazol-2-amine | R-116010 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H23N5S |
|---|
| Mol. Mass. | 377.506 |
|---|
| SMILES | C[C@H]([C@@H](c1ccc(Nc2nc3ccccc3s2)cc1)n1ccnc1)N(C)C |r| |
|---|
| Structure | 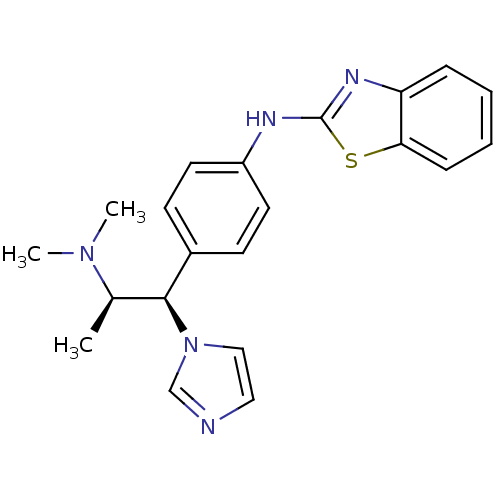
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gomaa, MS; Bridgens, CE; Veal, GJ; Redfern, CP; Brancale, A; Armstrong, JL; Simons, C Synthesis and biological evaluation of 3-(1H-imidazol- and triazol-1-yl)-2,2-dimethyl-3-[4-(naphthalen-2-ylamino)phenyl]propyl derivatives as small molecule inhibitors of retinoic acid 4-hydroxylase (CYP26). J Med Chem54:6803-11 (2011) [PubMed] Article
Gomaa, MS; Bridgens, CE; Veal, GJ; Redfern, CP; Brancale, A; Armstrong, JL; Simons, C Synthesis and biological evaluation of 3-(1H-imidazol- and triazol-1-yl)-2,2-dimethyl-3-[4-(naphthalen-2-ylamino)phenyl]propyl derivatives as small molecule inhibitors of retinoic acid 4-hydroxylase (CYP26). J Med Chem54:6803-11 (2011) [PubMed] Article