| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50354138 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_770526 (CHEMBL1839229) |
|---|
| IC50 | 8400±n/a nM |
|---|
| Citation |  Demont, EH; Arpino, S; Bit, RA; Campbell, CA; Deeks, N; Desai, S; Dowell, SJ; Gaskin, P; Gray, JR; Harrison, LA; Haynes, A; Heightman, TD; Holmes, DS; Humphreys, PG; Kumar, U; Morse, MA; Osborne, GJ; Panchal, T; Philpott, KL; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of a brain-penetrant S1P3-sparing direct agonist of the S1P¿? and S1P5 receptors efficacious at low oral dose. J Med Chem54:6724-33 (2011) [PubMed] Article Demont, EH; Arpino, S; Bit, RA; Campbell, CA; Deeks, N; Desai, S; Dowell, SJ; Gaskin, P; Gray, JR; Harrison, LA; Haynes, A; Heightman, TD; Holmes, DS; Humphreys, PG; Kumar, U; Morse, MA; Osborne, GJ; Panchal, T; Philpott, KL; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of a brain-penetrant S1P3-sparing direct agonist of the S1P¿? and S1P5 receptors efficacious at low oral dose. J Med Chem54:6724-33 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50354138 |
|---|
| n/a |
|---|
| Name | BDBM50354138 |
|---|
| Synonyms: | CHEMBL1836215 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H28N4O4 |
|---|
| Mol. Mass. | 448.5142 |
|---|
| SMILES | CC(C)Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc2CN(CCc2c1C)C(CO)CO |
|---|
| Structure | 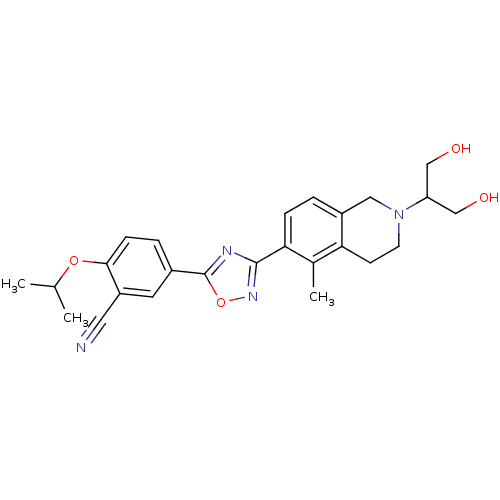
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Demont, EH; Arpino, S; Bit, RA; Campbell, CA; Deeks, N; Desai, S; Dowell, SJ; Gaskin, P; Gray, JR; Harrison, LA; Haynes, A; Heightman, TD; Holmes, DS; Humphreys, PG; Kumar, U; Morse, MA; Osborne, GJ; Panchal, T; Philpott, KL; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of a brain-penetrant S1P3-sparing direct agonist of the S1P¿? and S1P5 receptors efficacious at low oral dose. J Med Chem54:6724-33 (2011) [PubMed] Article
Demont, EH; Arpino, S; Bit, RA; Campbell, CA; Deeks, N; Desai, S; Dowell, SJ; Gaskin, P; Gray, JR; Harrison, LA; Haynes, A; Heightman, TD; Holmes, DS; Humphreys, PG; Kumar, U; Morse, MA; Osborne, GJ; Panchal, T; Philpott, KL; Taylor, S; Watson, R; Willis, R; Witherington, J Discovery of a brain-penetrant S1P3-sparing direct agonist of the S1P¿? and S1P5 receptors efficacious at low oral dose. J Med Chem54:6724-33 (2011) [PubMed] Article