| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase ABL1 |
|---|
| Ligand | BDBM13535 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_774464 (CHEMBL1908681) |
|---|
| Kd | >10000±n/a nM |
|---|
| Citation |  Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase ABL1 |
|---|
| Name: | Tyrosine-protein kinase ABL1 |
|---|
| Synonyms: | ABL | ABL1 | ABL1_HUMAN | Abelson murine leukemia viral oncogene homolog 1 | JTK7 | Proto-oncogene c-Abl | Proto-oncogene tyrosine-protein kinase ABL1 | Tyrosine-protein kinase (ABL) | Tyrosine-protein kinase ABL | Tyrosine-protein kinase ABL1 (ABL) | V-abl Abelson murine leukemia viral oncogene homolog 1 | c-ABL | p150 | tyrosine-protein kinase ABL1 isoform a |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 122897.30 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00519 |
|---|
| Residue: | 1130 |
|---|
| Sequence: | MLEICLKLVGCKSKKGLSSSSSCYLEEALQRPVASDFEPQGLSEAARWNSKENLLAGPSE
NDPNLFVALYDFVASGDNTLSITKGEKLRVLGYNHNGEWCEAQTKNGQGWVPSNYITPVN
SLEKHSWYHGPVSRNAAEYLLSSGINGSFLVRESESSPGQRSISLRYEGRVYHYRINTAS
DGKLYVSSESRFNTLAELVHHHSTVADGLITTLHYPAPKRNKPTVYGVSPNYDKWEMERT
DITMKHKLGGGQYGEVYEGVWKKYSLTVAVKTLKEDTMEVEEFLKEAAVMKEIKHPNLVQ
LLGVCTREPPFYIITEFMTYGNLLDYLRECNRQEVNAVVLLYMATQISSAMEYLEKKNFI
HRDLAARNCLVGENHLVKVADFGLSRLMTGDTYTAHAGAKFPIKWTAPESLAYNKFSIKS
DVWAFGVLLWEIATYGMSPYPGIDLSQVYELLEKDYRMERPEGCPEKVYELMRACWQWNP
SDRPSFAEIHQAFETMFQESSISDEVEKELGKQGVRGAVSTLLQAPELPTKTRTSRRAAE
HRDTTDVPEMPHSKGQGESDPLDHEPAVSPLLPRKERGPPEGGLNEDERLLPKDKKTNLF
SALIKKKKKTAPTPPKRSSSFREMDGQPERRGAGEEEGRDISNGALAFTPLDTADPAKSP
KPSNGAGVPNGALRESGGSGFRSPHLWKKSSTLTSSRLATGEEEGGGSSSKRFLRSCSAS
CVPHGAKDTEWRSVTLPRDLQSTGRQFDSSTFGGHKSEKPALPRKRAGENRSDQVTRGTV
TPPPRLVKKNEEAADEVFKDIMESSPGSSPPNLTPKPLRRQVTVAPASGLPHKEEAGKGS
ALGTPAAAEPVTPTSKAGSGAPGGTSKGPAEESRVRRHKHSSESPGRDKGKLSRLKPAPP
PPPAASAGKAGGKPSQSPSQEAAGEAVLGAKTKATSLVDAVNSDAAKPSQPGEGLKKPVL
PATPKPQSAKPSGTPISPAPVPSTLPSASSALAGDQPSSTAFIPLISTRVSLRKTRQPPE
RIASGAITKGVVLDSTEALCLAISRNSEQMASHSAVLEAGKNLYTFCVSYVDSIQQMRNK
FAFREAINKLENNLRELQICPATAGSGPAATQDFSKLLSSVKEISDIVQR
|
|
|
|---|
| BDBM13535 |
|---|
| n/a |
|---|
| Name | BDBM13535 |
|---|
| Synonyms: | 4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl]-N-(4-propan-2-yloxyphenyl)piperazine-1-carboxamide | 4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin-4-yl]-N-[4-(1-methylethoxy)phenyl]piperazine-1-carboxamide | 4-[6-methoxy-7-[3-(1-piperidinyl)propoxy]-4-quinazolinyl]-N-(4-propan-2-yloxyphenyl)-1-piperazinecarboxamide | 4-{6-methoxy-7-[3-(piperidin-1-yl)propoxy]quinazolin-4-yl}-N-[4-(propan-2-yloxy)phenyl]piperazine-1-carboxamide | CHEMBL124660 | MLN-518 | MLN518 | N-(4-isopropoxyphenyl)-4-[6-methoxy-7-(3-piperidinopropoxy)quinazolin-4-yl]piperazine-1-carboxamide | cid_3038522 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H42N6O4 |
|---|
| Mol. Mass. | 562.703 |
|---|
| SMILES | COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 |
|---|
| Structure | 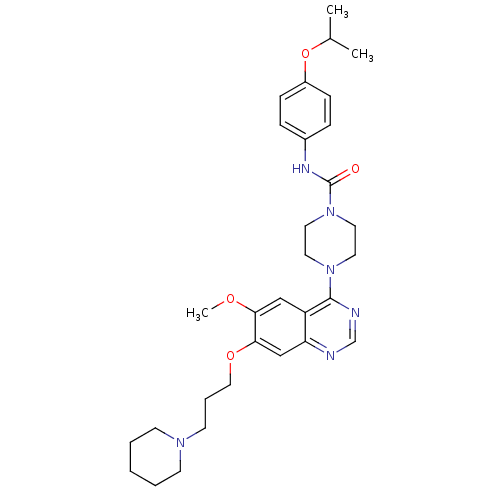
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article
Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article