| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase ABL1 |
|---|
| Ligand | BDBM50161957 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_774466 (CHEMBL1908683) |
|---|
| Kd | 7100±n/a nM |
|---|
| Citation |  Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase ABL1 |
|---|
| Name: | Tyrosine-protein kinase ABL1 |
|---|
| Synonyms: | ABL | ABL1 | ABL1_HUMAN | Abelson murine leukemia viral oncogene homolog 1 | JTK7 | Proto-oncogene c-Abl | Proto-oncogene tyrosine-protein kinase ABL1 | Tyrosine-protein kinase (ABL) | Tyrosine-protein kinase ABL | Tyrosine-protein kinase ABL1 (ABL) | V-abl Abelson murine leukemia viral oncogene homolog 1 | c-ABL | p150 | tyrosine-protein kinase ABL1 isoform a |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 122897.30 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00519 |
|---|
| Residue: | 1130 |
|---|
| Sequence: | MLEICLKLVGCKSKKGLSSSSSCYLEEALQRPVASDFEPQGLSEAARWNSKENLLAGPSE
NDPNLFVALYDFVASGDNTLSITKGEKLRVLGYNHNGEWCEAQTKNGQGWVPSNYITPVN
SLEKHSWYHGPVSRNAAEYLLSSGINGSFLVRESESSPGQRSISLRYEGRVYHYRINTAS
DGKLYVSSESRFNTLAELVHHHSTVADGLITTLHYPAPKRNKPTVYGVSPNYDKWEMERT
DITMKHKLGGGQYGEVYEGVWKKYSLTVAVKTLKEDTMEVEEFLKEAAVMKEIKHPNLVQ
LLGVCTREPPFYIITEFMTYGNLLDYLRECNRQEVNAVVLLYMATQISSAMEYLEKKNFI
HRDLAARNCLVGENHLVKVADFGLSRLMTGDTYTAHAGAKFPIKWTAPESLAYNKFSIKS
DVWAFGVLLWEIATYGMSPYPGIDLSQVYELLEKDYRMERPEGCPEKVYELMRACWQWNP
SDRPSFAEIHQAFETMFQESSISDEVEKELGKQGVRGAVSTLLQAPELPTKTRTSRRAAE
HRDTTDVPEMPHSKGQGESDPLDHEPAVSPLLPRKERGPPEGGLNEDERLLPKDKKTNLF
SALIKKKKKTAPTPPKRSSSFREMDGQPERRGAGEEEGRDISNGALAFTPLDTADPAKSP
KPSNGAGVPNGALRESGGSGFRSPHLWKKSSTLTSSRLATGEEEGGGSSSKRFLRSCSAS
CVPHGAKDTEWRSVTLPRDLQSTGRQFDSSTFGGHKSEKPALPRKRAGENRSDQVTRGTV
TPPPRLVKKNEEAADEVFKDIMESSPGSSPPNLTPKPLRRQVTVAPASGLPHKEEAGKGS
ALGTPAAAEPVTPTSKAGSGAPGGTSKGPAEESRVRRHKHSSESPGRDKGKLSRLKPAPP
PPPAASAGKAGGKPSQSPSQEAAGEAVLGAKTKATSLVDAVNSDAAKPSQPGEGLKKPVL
PATPKPQSAKPSGTPISPAPVPSTLPSASSALAGDQPSSTAFIPLISTRVSLRKTRQPPE
RIASGAITKGVVLDSTEALCLAISRNSEQMASHSAVLEAGKNLYTFCVSYVDSIQQMRNK
FAFREAINKLENNLRELQICPATAGSGPAATQDFSKLLSSVKEISDIVQR
|
|
|
|---|
| BDBM50161957 |
|---|
| n/a |
|---|
| Name | BDBM50161957 |
|---|
| Synonyms: | 4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(pyridin-2-ylmethoxy)-phenylamino]-3-cyano-7-ethoxy-quinolin-6-yl}-amide | CHEMBL180022 | HKI-272 | N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide | N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)butanamide | NERATINIB | US10822334, Compound Neratinib | US20230382923, Compound Neratinib |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H29ClN6O3 |
|---|
| Mol. Mass. | 557.043 |
|---|
| SMILES | CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C |
|---|
| Structure | 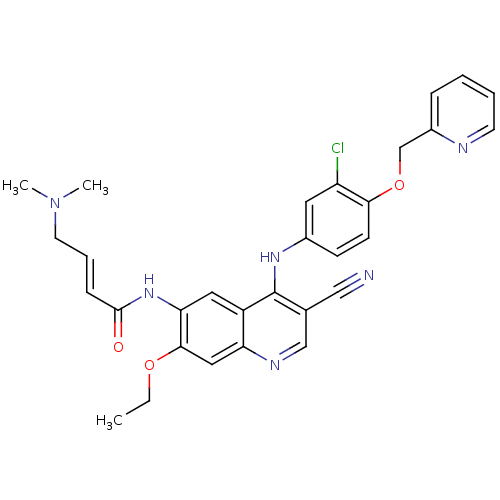
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article
Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article