| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Epithelial discoidin domain-containing receptor 1 |
|---|
| Ligand | BDBM50332294 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_774330 (CHEMBL1908547) |
|---|
| Kd | 1900±n/a nM |
|---|
| Citation |  Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Epithelial discoidin domain-containing receptor 1 |
|---|
| Name: | Epithelial discoidin domain-containing receptor 1 |
|---|
| Synonyms: | CAK | DDR1 | DDR1_HUMAN | Discoidin domain receptor 1 (DDR1) | Discoidin domain-containing receptor 1 (DDR1) | Discoidin receptor tyrosine kinase | EDDR1 | Epithelial discoidin domain receptor 1 | Epithelial discoidin domain receptor 1 (DDR1) | Epithelial discoidin domain-containing receptor 1 | Epithelial discoidin domain-containing receptor 1 (DDR1) | HGK2 | Mammary carcinoma kinase 10 | NEP | NTRK4 | PTK3A | Protein-tyrosine kinase RTK 6 | RTK6 | TRKE | Tyrosine-protein kinase CAK |
|---|
| Type: | Tyrosine-protein kinase |
|---|
| Mol. Mass.: | 101130.02 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q08345 |
|---|
| Residue: | 913 |
|---|
| Sequence: | MGPEALSSLLLLLLVASGDADMKGHFDPAKCRYALGMQDRTIPDSDISASSSWSDSTAAR
HSRLESSDGDGAWCPAGSVFPKEEEYLQVDLQRLHLVALVGTQGRHAGGLGKEFSRSYRL
RYSRDGRRWMGWKDRWGQEVISGNEDPEGVVLKDLGPPMVARLVRFYPRADRVMSVCLRV
ELYGCLWRDGLLSYTAPVGQTMYLSEAVYLNDSTYDGHTVGGLQYGGLGQLADGVVGLDD
FRKSQELRVWPGYDYVGWSNHSFSSGYVEMEFEFDRLRAFQAMQVHCNNMHTLGARLPGG
VECRFRRGPAMAWEGEPMRHNLGGNLGDPRARAVSVPLGGRVARFLQCRFLFAGPWLLFS
EISFISDVVNNSSPALGGTFPPAPWWPPGPPPTNFSSLELEPRGQQPVAKAEGSPTAILI
GCLVAIILLLLLIIALMLWRLHWRRLLSKAERRVLEEELTVHLSVPGDTILINNRPGPRE
PPPYQEPRPRGNPPHSAPCVPNGSALLLSNPAYRLLLATYARPPRGPGPPTPAWAKPTNT
QAYSGDYMEPEKPGAPLLPPPPQNSVPHYAEADIVTLQGVTGGNTYAVPALPPGAVGDGP
PRVDFPRSRLRFKEKLGEGQFGEVHLCEVDSPQDLVSLDFPLNVRKGHPLLVAVKILRPD
ATKNARNDFLKEVKIMSRLKDPNIIRLLGVCVQDDPLCMITDYMENGDLNQFLSAHQLED
KAAEGAPGDGQAAQGPTISYPMLLHVAAQIASGMRYLATLNFVHRDLATRNCLVGENFTI
KIADFGMSRNLYAGDYYRVQGRAVLPIRWMAWECILMGKFTTASDVWAFGVTLWEVLMLC
RAQPFGQLTDEQVIENAGEFFRDQGRQVYLSRPPACPQGLYELMLRCWSRESEQRPPFSQ
LHRFLAEDALNTV
|
|
|
|---|
| BDBM50332294 |
|---|
| n/a |
|---|
| Name | BDBM50332294 |
|---|
| Synonyms: | CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(pyrrolidin-1-yl)ethoxy)phenylamino)pyrimidin-4-ylamino)benzenesulfonamide | US10112907, Example 00018 | US10730860, TABLE 1.3 | US10766894, Compound TABLE 1.3 | US11203595, TABLE 1.3 | US11384069, Example T-5 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H36N6O3S |
|---|
| Mol. Mass. | 524.678 |
|---|
| SMILES | Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C |
|---|
| Structure | 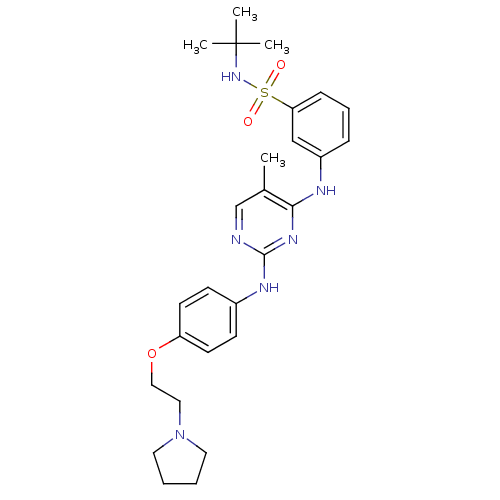
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article
Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol29:1046-51 (2011) [PubMed] Article