| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetyl-CoA carboxylase 2 |
|---|
| Ligand | BDBM50189617 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_775975 (CHEMBL1912973) |
|---|
| IC50 | 34±n/a nM |
|---|
| Citation |  Yamashita, T; Kamata, M; Endo, S; Yamamoto, M; Kakegawa, K; Watanabe, H; Miwa, K; Yamano, T; Funata, M; Sakamoto, J; Tani, A; Mol, CD; Zou, H; Dougan, DR; Sang, B; Snell, G; Fukatsu, K Design, synthesis, and structure-activity relationships of spirolactones bearing 2-ureidobenzothiophene as acetyl-CoA carboxylases inhibitors. Bioorg Med Chem Lett21:6314-8 (2011) [PubMed] Article Yamashita, T; Kamata, M; Endo, S; Yamamoto, M; Kakegawa, K; Watanabe, H; Miwa, K; Yamano, T; Funata, M; Sakamoto, J; Tani, A; Mol, CD; Zou, H; Dougan, DR; Sang, B; Snell, G; Fukatsu, K Design, synthesis, and structure-activity relationships of spirolactones bearing 2-ureidobenzothiophene as acetyl-CoA carboxylases inhibitors. Bioorg Med Chem Lett21:6314-8 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetyl-CoA carboxylase 2 |
|---|
| Name: | Acetyl-CoA carboxylase 2 |
|---|
| Synonyms: | ACACB | ACACB_HUMAN | ACC-beta | ACC2 | ACCB | Acetyl-CoA carboxylase | Acetyl-CoA carboxylase 2 | Acetyl-CoA carboxylase 2 (ACC) | Acetyl-CoA carboxylase 2 (ACC2) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 276535.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O00763 |
|---|
| Residue: | 2458 |
|---|
| Sequence: | MVLLLCLSCLIFSCLTFSWLKIWGKMTDSKPITKSKSEANLIPSQEPFPASDNSGETPQR
NGEGHTLPKTPSQAEPASHKGPKDAGRRRNSLPPSHQKPPRNPLSSSDAAPSPELQANGT
GTQGLEATDTNGLSSSARPQGQQAGSPSKEDKKQANIKRQLMTNFILGSFDDYSSDEDSV
AGSSRESTRKGSRASLGALSLEAYLTTGEAETRVPTMRPSMSGLHLVKRGREHKKLDLHR
DFTVASPAEFVTRFGGDRVIEKVLIANNGIAAVKCMRSIRRWAYEMFRNERAIRFVVMVT
PEDLKANAEYIKMADHYVPVPGGPNNNNYANVELIVDIAKRIPVQAVWAGWGHASENPKL
PELLCKNGVAFLGPPSEAMWALGDKIASTVVAQTLQVPTLPWSGSGLTVEWTEDDLQQGK
RISVPEDVYDKGCVKDVDEGLEAAERIGFPLMIKASEGGGGKGIRKAESAEDFPILFRQV
QSEIPGSPIFLMKLAQHARHLEVQILADQYGNAVSLFGRDCSIQRRHQKIVEEAPATIAP
LAIFEFMEQCAIRLAKTVGYVSAGTVEYLYSQDGSFHFLELNPRLQVEHPCTEMIADVNL
PAAQLQIAMGVPLHRLKDIRLLYGESPWGVTPISFETPSNPPLARGHVIAARITSENPDE
GFKPSSGTVQELNFRSSKNVWGYFSVAATGGLHEFADSQFGHCFSWGENREEAISNMVVA
LKELSIRGDFRTTVEYLINLLETESFQNNDIDTGWLDYLIAEKVQAEKPDIMLGVVCGAL
NVADAMFRTCMTDFLHSLERGQVLPADSLLNLVDVELIYGGVKYILKVARQSLTMFVLIM
NGCHIEIDAHRLNDGGLLLSYNGNSYTTYMKEEVDSYRITIGNKTCVFEKENDPTVLRSP
SAGKLTQYTVEDGGHVEAGSSYAEMEVMKMIMTLNVQERGRVKYIKRPGAVLEAGCVVAR
LELDDPSKVHPAEPFTGELPAQQTLPILGEKLHQVFHSVLENLTNVMSGFCLPEPVFSIK
LKEWVQKLMMTLRHPSLPLLELQEIMTSVAGRIPAPVEKSVRRVMAQYASNITSVLCQFP
SQQIATILDCHAATLQRKADREVFFINTQSIVQLVQRYRSGIRGYMKTVVLDLLRRYLRV
EHHFQQAHYDKCVINLREQFKPDMSQVLDCIFSHAQVAKKNQLVIMLIDELCGPDPSLSD
ELISILNELTQLSKSEHCKVALRARQILIASHLPSYELRHNQVESIFLSAIDMYGHQFCP
ENLKKLILSETTIFDVLPTFFYHANKVVCMASLEVYVRRGYIAYELNSLQHRQLPDGTCV
VEFQFMLPSSHPNRMTVPISITNPDLLRHSTELFMDSGFSPLCQRMGAMVAFRRFEDFTR
NFDEVISCFANVPKDTPLFSEARTSLYSEDDCKSLREEPIHILNVSIQCADHLEDEALVP
ILRTFVQSKKNILVDYGLRRITFLIAQEKEFPKFFTFRARDEFAEDRIYRHLEPALAFQL
ELNRMRNFDLTAVPCANHKMHLYLGAAKVKEGVEVTDHRFFIRAIIRHSDLITKEASFEY
LQNEGERLLLEAMDELEVAFNNTSVRTDCNHIFLNFVPTVIMDPFKIEESVRYMVMRYGS
RLWKLRVLQAEVKINIRQTTTGSAVPIRLFITNESGYYLDISLYKEVTDSRSGNIMFHSF
GNKQGPQHGMLINTPYVTKDLLQAKRFQAQTLGTTYIYDFPEMFRQALFKLWGSPDKYPK
DILTYTELVLDSQGQLVEMNRLPGGNEVGMVAFKMRFKTQEYPEGRDVIVIGNDITFRIG
SFGPGEDLLYLRASEMARAEGIPKIYVAANSGARIGMAEEIKHMFHVAWVDPEDPHKGFK
YLYLTPQDYTRISSLNSVHCKHIEEGGESRYMITDIIGKDDGLGVENLRGSGMIAGESSL
AYEEIVTISLVTCRAIGIGAYLVRLGQRVIQVENSHIILTGASALNKVLGREVYTSNNQL
GGVQIMHYNGVSHITVPDDFEGVYTILEWLSYMPKDNHSPVPIITPTDPIDREIEFLPSR
APYDPRWMLAGRPHPTLKGTWQSGFFDHGSFKEIMAPWAQTVVTGRARLGGIPVGVIAVE
TRTVEVAVPADPANLDSEAKIIQQAGQVWFPDSAYKTAQAVKDFNREKLPLMIFANWRGF
SGGMKDMYDQVLKFGAYIVDGLRQYKQPILIYIPPYAELRGGSWVVIDATINPLCIEMYA
DKESRGGVLEPEGTVEIKFRKKDLIKSMRRIDPAYKKLMEQLGEPDLSDKDRKDLEGRLK
AREDLLLPIYHQVAVQFADFHDTPGRMLEKGVISDILEWKTARTFLYWRLRRLLLEDQVK
QEILQASGELSHVHIQSMLRRWFVETEGAVKAYLWDNNQVVVQWLEQHWQAGDGPRSTIR
ENITYLKHDSVLKTIRGLVEENPEVAVDCVIYLSQHISPAERAQVVHLLSTMDSPAST
|
|
|
|---|
| BDBM50189617 |
|---|
| n/a |
|---|
| Name | BDBM50189617 |
|---|
| Synonyms: | (3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbonyl)-1,4'-bipiperidine | (3R)-1'-(anthracen-9-ylcarbonyl)-3-(morpholin-4-ylcarbonyl)-1,4'-bipiperidine | CHEMBL208943 | {(3R)-1'-(anthracen-9-ylcarbonyl)[1,4'-bipiperidin]-3-yl}(morpholin-4-yl)methanone |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H35N3O3 |
|---|
| Mol. Mass. | 485.6172 |
|---|
| SMILES | O=C([C@@H]1CCCN(C1)C1CCN(CC1)C(=O)c1c2ccccc2cc2ccccc12)N1CCOCC1 |r| |
|---|
| Structure | 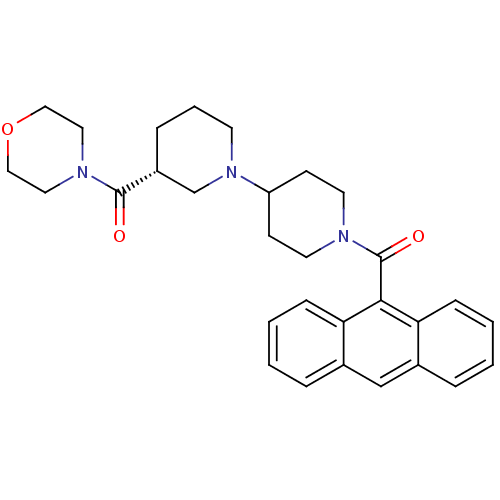
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yamashita, T; Kamata, M; Endo, S; Yamamoto, M; Kakegawa, K; Watanabe, H; Miwa, K; Yamano, T; Funata, M; Sakamoto, J; Tani, A; Mol, CD; Zou, H; Dougan, DR; Sang, B; Snell, G; Fukatsu, K Design, synthesis, and structure-activity relationships of spirolactones bearing 2-ureidobenzothiophene as acetyl-CoA carboxylases inhibitors. Bioorg Med Chem Lett21:6314-8 (2011) [PubMed] Article
Yamashita, T; Kamata, M; Endo, S; Yamamoto, M; Kakegawa, K; Watanabe, H; Miwa, K; Yamano, T; Funata, M; Sakamoto, J; Tani, A; Mol, CD; Zou, H; Dougan, DR; Sang, B; Snell, G; Fukatsu, K Design, synthesis, and structure-activity relationships of spirolactones bearing 2-ureidobenzothiophene as acetyl-CoA carboxylases inhibitors. Bioorg Med Chem Lett21:6314-8 (2011) [PubMed] Article