| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, brain |
|---|
| Ligand | BDBM50357690 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_785761 (CHEMBL1920781) |
|---|
| IC50 | 93±n/a nM |
|---|
| Citation |  Ramnauth, J; Speed, J; Maddaford, SP; Dove, P; Annedi, SC; Renton, P; Rakhit, S; Andrews, J; Silverman, S; Mladenova, G; Zinghini, S; Nair, S; Catalano, C; Lee, DK; De Felice, M; Porreca, F Design, synthesis, and biological evaluation of 3,4-dihydroquinolin-2(1H)-one and 1,2,3,4-tetrahydroquinoline-based selective human neuronal nitric oxide synthase (nNOS) inhibitors. J Med Chem54:5562-75 (2011) [PubMed] Article Ramnauth, J; Speed, J; Maddaford, SP; Dove, P; Annedi, SC; Renton, P; Rakhit, S; Andrews, J; Silverman, S; Mladenova, G; Zinghini, S; Nair, S; Catalano, C; Lee, DK; De Felice, M; Porreca, F Design, synthesis, and biological evaluation of 3,4-dihydroquinolin-2(1H)-one and 1,2,3,4-tetrahydroquinoline-based selective human neuronal nitric oxide synthase (nNOS) inhibitors. J Med Chem54:5562-75 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, brain |
|---|
| Name: | Nitric oxide synthase, brain |
|---|
| Synonyms: | Constitutive NOS | N-NOS | NC-NOS | NOS type I | NOS type I nNOS | NOS1 | NOS1_HUMAN | Neuronal NOS | Neuronal nitric oxide synthase | Nitric oxide synthase, brain (nNOS) | Nitric oxide synthase, neuronal (nNOS) | Peptidyl-cysteine S-nitrosylase NOS1 | bNOS | nNOS |
|---|
| Type: | Homodimer |
|---|
| Mol. Mass.: | 160985.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29475 |
|---|
| Residue: | 1434 |
|---|
| Sequence: | MEDHMFGVQQIQPNVISVRLFKRKVGGLGFLVKERVSKPPVIISDLIRGGAAEQSGLIQA
GDIILAVNGRPLVDLSYDSALEVLRGIASETHVVLILRGPEGFTTHLETTFTGDGTPKTI
RVTQPLGPPTKAVDLSHQPPAGKEQPLAVDGASGPGNGPQHAYDDGQEAGSLPHANGLAP
RPPGQDPAKKATRVSLQGRGENNELLKEIEPVLSLLTSGSRGVKGGAPAKAEMKDMGIQV
DRDLDGKSHKPLPLGVENDRVFNDLWGKGNVPVVLNNPYSEKEQPPTSGKQSPTKNGSPS
KCPRFLKVKNWETEVVLTDTLHLKSTLETGCTEYICMGSIMHPSQHARRPEDVRTKGQLF
PLAKEFIDQYYSSIKRFGSKAHMERLEEVNKEIDTTSTYQLKDTELIYGAKHAWRNASRC
VGRIQWSKLQVFDARDCTTAHGMFNYICNHVKYATNKGNLRSAITIFPQRTDGKHDFRVW
NSQLIRYAGYKQPDGSTLGDPANVQFTEICIQQGWKPPRGRFDVLPLLLQANGNDPELFQ
IPPELVLEVPIRHPKFEWFKDLGLKWYGLPAVSNMLLEIGGLEFSACPFSGWYMGTEIGV
RDYCDNSRYNILEEVAKKMNLDMRKTSSLWKDQALVEINIAVLYSFQSDKVTIVDHHSAT
ESFIKHMENEYRCRGGCPADWVWIVPPMSGSITPVFHQEMLNYRLTPSFEYQPDPWNTHV
WKGTNGTPTKRRAIGFKKLAEAVKFSAKLMGQAMAKRVKATILYATETGKSQAYAKTLCE
IFKHAFDAKVMSMEEYDIVHLEHETLVLVVTSTFGNGDPPENGEKFGCALMEMRHPNSVQ
EERKSYKVRFNSVSSYSDSQKSSGDGPDLRDNFESAGPLANVRFSVFGLGSRAYPHFCAF
GHAVDTLLEELGGERILKMREGDELCGQEEAFRTWAKKVFKAACDVFCVGDDVNIEKANN
SLISNDRSWKRNKFRLTFVAEAPELTQGLSNVHKKRVSAARLLSRQNLQSPKSSRSTIFV
RLHTNGSQELQYQPGDHLGVFPGNHEDLVNALIERLEDAPPVNQMVKVELLEERNTALGV
ISNWTDELRLPPCTIFQAFKYYLDITTPPTPLQLQQFASLATSEKEKQRLLVLSKGLQEY
EEWKWGKNPTIVEVLEEFPSIQMPATLLLTQLSLLQPRYYSISSSPDMYPDEVHLTVAIV
SYRTRDGEGPIHHGVCSSWLNRIQADELVPCFVRGAPSFHLPRNPQVPCILVGPGTGIAP
FRSFWQQRQFDIQHKGMNPCPMVLVFGCRQSKIDHIYREETLQAKNKGVFRELYTAYSRE
PDKPKKYVQDILQEQLAESVYRALKEQGGHIYVCGDVTMAADVLKAIQRIMTQQGKLSAE
DAGVFISRMRDDNRYHEDIFGVTLRTYEVTNRLRSESIAFIEESKKDTDEVFSS
|
|
|
|---|
| BDBM50357690 |
|---|
| n/a |
|---|
| Name | BDBM50357690 |
|---|
| Synonyms: | CHEMBL1915286 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H23FN4S |
|---|
| Mol. Mass. | 346.465 |
|---|
| SMILES | CN(C)CCN1CCCc2cc(cc(F)c12)N=C(N)c1cccs1 |w:16.17| |
|---|
| Structure | 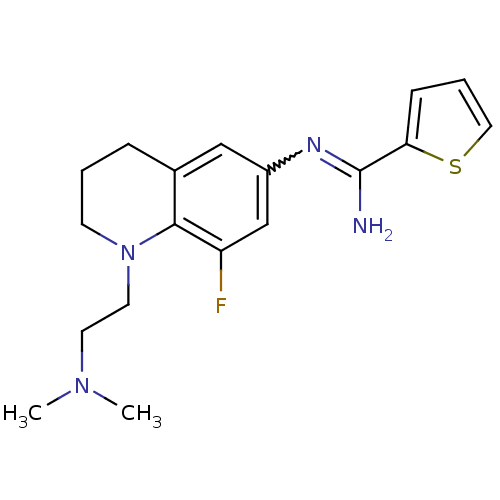
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ramnauth, J; Speed, J; Maddaford, SP; Dove, P; Annedi, SC; Renton, P; Rakhit, S; Andrews, J; Silverman, S; Mladenova, G; Zinghini, S; Nair, S; Catalano, C; Lee, DK; De Felice, M; Porreca, F Design, synthesis, and biological evaluation of 3,4-dihydroquinolin-2(1H)-one and 1,2,3,4-tetrahydroquinoline-based selective human neuronal nitric oxide synthase (nNOS) inhibitors. J Med Chem54:5562-75 (2011) [PubMed] Article
Ramnauth, J; Speed, J; Maddaford, SP; Dove, P; Annedi, SC; Renton, P; Rakhit, S; Andrews, J; Silverman, S; Mladenova, G; Zinghini, S; Nair, S; Catalano, C; Lee, DK; De Felice, M; Porreca, F Design, synthesis, and biological evaluation of 3,4-dihydroquinolin-2(1H)-one and 1,2,3,4-tetrahydroquinoline-based selective human neuronal nitric oxide synthase (nNOS) inhibitors. J Med Chem54:5562-75 (2011) [PubMed] Article