

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Ephrin type-A receptor 2 | ||
| Ligand | BDBM50357884 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_786755 (CHEMBL1919596) | ||
| IC50 | >10000±n/a nM | ||
| Citation |  Lumeras, W; Vidal, L; Vidal, B; Balagué, C; Orellana, A; Maldonado, M; Domínguez, M; Segarra, V; Caturla, F 1,7-Naphthyridine 1-oxides as novel potent and selective inhibitors of p38 mitogen activated protein kinase. J Med Chem54:7899-910 (2011) [PubMed] Article Lumeras, W; Vidal, L; Vidal, B; Balagué, C; Orellana, A; Maldonado, M; Domínguez, M; Segarra, V; Caturla, F 1,7-Naphthyridine 1-oxides as novel potent and selective inhibitors of p38 mitogen activated protein kinase. J Med Chem54:7899-910 (2011) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Ephrin type-A receptor 2 | |||
| Name: | Ephrin type-A receptor 2 | ||
| Synonyms: | ECK | EPHA2 | EPHA2_HUMAN | Ephrin receptor | Epithelial cell kinase | Tyrosine-protein kinase receptor ECK | ||
| Type: | PROTEIN | ||
| Mol. Mass.: | 108260.70 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | ChEMBL_1505248 | ||
| Residue: | 976 | ||
| Sequence: |
| ||
| BDBM50357884 | |||
| n/a | |||
| Name | BDBM50357884 | ||
| Synonyms: | CHEMBL1916359 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H10Cl3FN2O | ||
| Mol. Mass. | 419.664 | ||
| SMILES | [O-][n+]1ccc2c(ccnc2c1-c1c(Cl)cccc1Cl)-c1ccc(F)cc1Cl |(12.75,-22.82,;14.08,-22.05,;14.08,-20.51,;15.41,-19.74,;16.75,-20.5,;18.08,-19.73,;19.42,-20.51,;19.42,-22.05,;18.08,-22.82,;16.74,-22.05,;15.42,-22.83,;15.42,-24.36,;14.08,-25.13,;12.75,-24.36,;14.09,-26.67,;15.42,-27.44,;16.76,-26.66,;16.75,-25.12,;18.08,-24.34,;18.08,-18.2,;19.42,-17.43,;19.42,-15.89,;18.08,-15.12,;18.08,-13.58,;16.75,-15.89,;16.75,-17.43,;15.42,-18.2,)| | ||
| Structure | 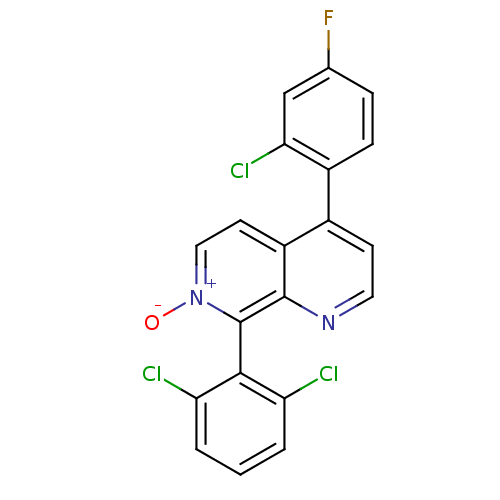
| ||