| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50358174 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_788860 (CHEMBL1924930) |
|---|
| IC50 | >30000±n/a nM |
|---|
| Citation |  Jones, CK; Engers, DW; Thompson, AD; Field, JR; Blobaum, AL; Lindsley, SR; Zhou, Y; Gogliotti, RD; Jadhav, S; Zamorano, R; Bogenpohl, J; Smith, Y; Morrison, R; Daniels, JS; Weaver, CD; Conn, PJ; Lindsley, CW; Niswender, CM; Hopkins, CR Discovery, synthesis, and structure-activity relationship development of a series of N-4-(2,5-dioxopyrrolidin-1-yl)phenylpicolinamides (VU0400195, ML182): characterization of a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu(4)) with oral efficacy in an antiparkin J Med Chem54:7639-47 (2011) [PubMed] Article Jones, CK; Engers, DW; Thompson, AD; Field, JR; Blobaum, AL; Lindsley, SR; Zhou, Y; Gogliotti, RD; Jadhav, S; Zamorano, R; Bogenpohl, J; Smith, Y; Morrison, R; Daniels, JS; Weaver, CD; Conn, PJ; Lindsley, CW; Niswender, CM; Hopkins, CR Discovery, synthesis, and structure-activity relationship development of a series of N-4-(2,5-dioxopyrrolidin-1-yl)phenylpicolinamides (VU0400195, ML182): characterization of a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu(4)) with oral efficacy in an antiparkin J Med Chem54:7639-47 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50358174 |
|---|
| n/a |
|---|
| Name | BDBM50358174 |
|---|
| Synonyms: | CHEMBL1921855 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H13N3O5S2 |
|---|
| Mol. Mass. | 415.443 |
|---|
| SMILES | O=C(Nc1ccc(cc1)N1S(=O)(=O)c2ccccc2S1(=O)=O)c1ccccn1 |
|---|
| Structure | 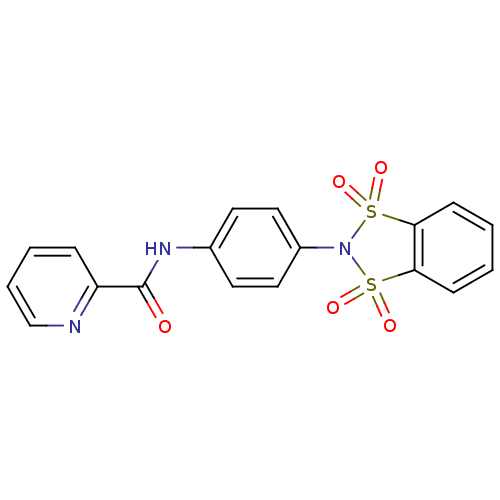
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jones, CK; Engers, DW; Thompson, AD; Field, JR; Blobaum, AL; Lindsley, SR; Zhou, Y; Gogliotti, RD; Jadhav, S; Zamorano, R; Bogenpohl, J; Smith, Y; Morrison, R; Daniels, JS; Weaver, CD; Conn, PJ; Lindsley, CW; Niswender, CM; Hopkins, CR Discovery, synthesis, and structure-activity relationship development of a series of N-4-(2,5-dioxopyrrolidin-1-yl)phenylpicolinamides (VU0400195, ML182): characterization of a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu(4)) with oral efficacy in an antiparkin J Med Chem54:7639-47 (2011) [PubMed] Article
Jones, CK; Engers, DW; Thompson, AD; Field, JR; Blobaum, AL; Lindsley, SR; Zhou, Y; Gogliotti, RD; Jadhav, S; Zamorano, R; Bogenpohl, J; Smith, Y; Morrison, R; Daniels, JS; Weaver, CD; Conn, PJ; Lindsley, CW; Niswender, CM; Hopkins, CR Discovery, synthesis, and structure-activity relationship development of a series of N-4-(2,5-dioxopyrrolidin-1-yl)phenylpicolinamides (VU0400195, ML182): characterization of a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu(4)) with oral efficacy in an antiparkin J Med Chem54:7639-47 (2011) [PubMed] Article