| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neuraminidase |
|---|
| Ligand | BDBM5024 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_794312 (CHEMBL1932234) |
|---|
| Ki | 0.83±n/a nM |
|---|
| Citation |  Bromba, CM; Mason, JW; Brant, MG; Chan, T; Lunke, MD; Petric, M; Boulanger, MJ; Wulff, JE The de-guanidinylated derivative of peramivir remains a potent inhibitor of influenza neuraminidase. Bioorg Med Chem Lett21:7137-41 (2011) [PubMed] Article Bromba, CM; Mason, JW; Brant, MG; Chan, T; Lunke, MD; Petric, M; Boulanger, MJ; Wulff, JE The de-guanidinylated derivative of peramivir remains a potent inhibitor of influenza neuraminidase. Bioorg Med Chem Lett21:7137-41 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Neuraminidase |
|---|
| Name: | Neuraminidase |
|---|
| Synonyms: | NA | NRAM_I18A0 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 51401.00 |
|---|
| Organism: | Influenza A virus (strain A/Brevig Mission/1/1918 H1N1) (Influenza Avirus (strain A/South Carolina/1/1918 H1N1)) |
|---|
| Description: | ChEMBL_794312 |
|---|
| Residue: | 469 |
|---|
| Sequence: | MNPNQKIITIGSICMVVGIISLILQIGNIISIWVSHSIQTGNQNHPETCNQSIITYENNT
WVNQTYVNISNTNVVAGQDATSVILTGNSSLCPISGWAIYSKDNGIRIGSKGDVFVIREP
FISCSHLECRTFFLTQGALLNDKHSNGTVKDRSPYRTLMSCPVGEAPSPYNSRFESVAWS
ASACHDGMGWLTIGISGPDNGAVAVLKYNGIITDTIKSWRNNILRTQESECACVNGSCFT
IMTDGPSNGQASYKILKIEKGKVTKSIELNAPNYHYEECSCYPDTGKVMCVCRDNWHGSN
RPWVSFDQNLDYQIGYICSGVFGDNPRPNDGTGSCGPVSSNGANGIKGFSFRYDNGVWIG
RTKSTSSRSGFEMIWDPNGWTETDSSFSVRQDIVAITDWSGYSGSFVQHPELTGLDCMRP
CFWVELIRGQPKENTIWTSGSSISFCGVNSDTVGWSWPDGAELPFSIDK
|
|
|
|---|
| BDBM5024 |
|---|
| n/a |
|---|
| Name | BDBM5024 |
|---|
| Synonyms: | (-)-(1S,2S,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylbutyl]-4-{[amino(imino)methyl]amino}-2-hydroxycyclopentanecarboxylic Acid | (1S,2S,3R,4R)-4-carbamimidamido-3-[(1S)-1-acetamido-2-ethylbutyl]-2-hydroxycyclopentane-1-carboxylic acid | BCX-1812 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H28N4O4 |
|---|
| Mol. Mass. | 328.4072 |
|---|
| SMILES | [H][C@](NC(C)=O)(C(CC)CC)[C@@H]1[C@H](O)[C@H](C[C@H]1N=C(N)N)C(O)=O |r| |
|---|
| Structure | 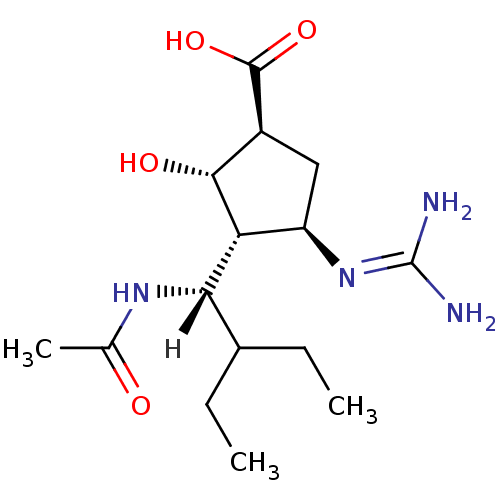
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bromba, CM; Mason, JW; Brant, MG; Chan, T; Lunke, MD; Petric, M; Boulanger, MJ; Wulff, JE The de-guanidinylated derivative of peramivir remains a potent inhibitor of influenza neuraminidase. Bioorg Med Chem Lett21:7137-41 (2011) [PubMed] Article
Bromba, CM; Mason, JW; Brant, MG; Chan, T; Lunke, MD; Petric, M; Boulanger, MJ; Wulff, JE The de-guanidinylated derivative of peramivir remains a potent inhibitor of influenza neuraminidase. Bioorg Med Chem Lett21:7137-41 (2011) [PubMed] Article