| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen synthase kinase-3 beta |
|---|
| Ligand | BDBM50361089 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_795090 (CHEMBL1936516) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Caruso, M; Valsasina, B; Ballinari, D; Bertrand, J; Brasca, MG; Caldarelli, M; Cappella, P; Fiorentini, F; Gianellini, LM; Scolaro, A; Beria, I 5-(2-amino-pyrimidin-4-yl)-1H-pyrrole and 2-(2-amino-pyrimidin-4-yl)-1,5,6,7-tetrahydro-pyrrolo[3,2-c]pyridin-4-one derivatives as new classes of selective and orally available Polo-like kinase 1 inhibitors. Bioorg Med Chem Lett22:96-101 (2011) [PubMed] Article Caruso, M; Valsasina, B; Ballinari, D; Bertrand, J; Brasca, MG; Caldarelli, M; Cappella, P; Fiorentini, F; Gianellini, LM; Scolaro, A; Beria, I 5-(2-amino-pyrimidin-4-yl)-1H-pyrrole and 2-(2-amino-pyrimidin-4-yl)-1,5,6,7-tetrahydro-pyrrolo[3,2-c]pyridin-4-one derivatives as new classes of selective and orally available Polo-like kinase 1 inhibitors. Bioorg Med Chem Lett22:96-101 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen synthase kinase-3 beta |
|---|
| Name: | Glycogen synthase kinase-3 beta |
|---|
| Synonyms: | GSK-3 beta | GSK-3, beta | GSK3B | GSK3B_HUMAN | Glycogen synthase kinase 3 beta (GSK3B) | Glycogen synthase kinase 3-beta (GSK3B) | Glycogen synthase kinase-3 beta (GSK-3B) | Glycogen synthase kinase-3 beta (GSK3 Beta) | Glycogen synthase kinase-3 beta (GSK3B) | Glycogen synthase kinase-3B (GSK-3B) | Glycogen synthase kinase-3beta (GSK3B) | Serine/threonine-protein kinase GSK3B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 46756.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49841 |
|---|
| Residue: | 420 |
|---|
| Sequence: | MSGRPRTTSFAESCKPVQQPSAFGSMKVSRDKDGSKVTTVVATPGQGPDRPQEVSYTDTK
VIGNGSFGVVYQAKLCDSGELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFYSSG
EKKDEVYLNLVLDYVPETVYRVARHYSRAKQTLPVIYVKLYMYQLFRSLAYIHSFGICHR
DIKPQNLLLDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSSIDV
WSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIKAHP
WTKVFRPRTPPEAIALCSRLLEYTPTARLTPLEACAHSFFDELRDPNVKLPNGRDTPALF
NFTTQELSSNPPLATILIPPHARIQAAASTPTNATAASDANTGDRGQTNNAASASASNST
|
|
|
|---|
| BDBM50361089 |
|---|
| n/a |
|---|
| Name | BDBM50361089 |
|---|
| Synonyms: | CHEMBL1933576 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H24F3N7O2 |
|---|
| Mol. Mass. | 475.4669 |
|---|
| SMILES | CN1CCN(CC1)c1ccc(OC(F)(F)F)c(Nc2nccc(n2)-c2cc(cn2C)C(N)=O)c1 |
|---|
| Structure | 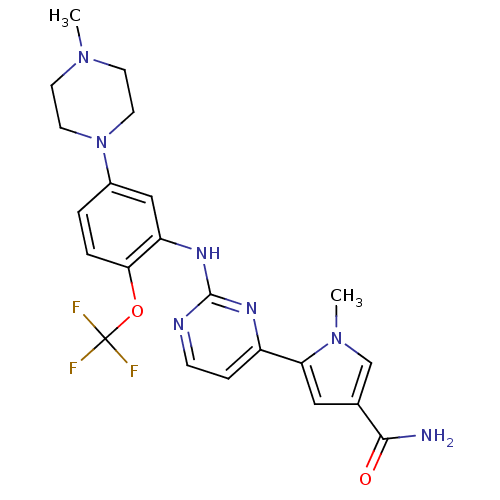
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Caruso, M; Valsasina, B; Ballinari, D; Bertrand, J; Brasca, MG; Caldarelli, M; Cappella, P; Fiorentini, F; Gianellini, LM; Scolaro, A; Beria, I 5-(2-amino-pyrimidin-4-yl)-1H-pyrrole and 2-(2-amino-pyrimidin-4-yl)-1,5,6,7-tetrahydro-pyrrolo[3,2-c]pyridin-4-one derivatives as new classes of selective and orally available Polo-like kinase 1 inhibitors. Bioorg Med Chem Lett22:96-101 (2011) [PubMed] Article
Caruso, M; Valsasina, B; Ballinari, D; Bertrand, J; Brasca, MG; Caldarelli, M; Cappella, P; Fiorentini, F; Gianellini, LM; Scolaro, A; Beria, I 5-(2-amino-pyrimidin-4-yl)-1H-pyrrole and 2-(2-amino-pyrimidin-4-yl)-1,5,6,7-tetrahydro-pyrrolo[3,2-c]pyridin-4-one derivatives as new classes of selective and orally available Polo-like kinase 1 inhibitors. Bioorg Med Chem Lett22:96-101 (2011) [PubMed] Article