| Reaction Details |
|---|
 | Report a problem with these data |
| Target | P2Y purinoceptor 2 |
|---|
| Ligand | BDBM50205413 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_805342 (CHEMBL1955235) |
|---|
| EC50 | 60±n/a nM |
|---|
| Citation |  Yelovitch, S; Camden, J; Weisman, GA; Fischer, B Boranophosphate isoster controls P2Y-receptor subtype selectivity and metabolic stability of dinucleoside polyphosphate analogues. J Med Chem55:437-48 (2012) [PubMed] Article Yelovitch, S; Camden, J; Weisman, GA; Fischer, B Boranophosphate isoster controls P2Y-receptor subtype selectivity and metabolic stability of dinucleoside polyphosphate analogues. J Med Chem55:437-48 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| P2Y purinoceptor 2 |
|---|
| Name: | P2Y purinoceptor 2 |
|---|
| Synonyms: | ATP receptor | P2RU1 | P2RY2 | P2RY2_HUMAN | P2U purinoceptor 1 | P2U1 | P2Y purinoceptor 2 | P2Y2 | Purinergic receptor | Purinergic receptor P2Y2 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 42299.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1455361 |
|---|
| Residue: | 377 |
|---|
| Sequence: | MAADLGPWNDTINGTWDGDELGYRCRFNEDFKYVLLPVSYGVVCVPGLCLNAVALYIFLC
RLKTWNASTTYMFHLAVSDALYAASLPLLVYYYARGDHWPFSTVLCKLVRFLFYTNLYCS
ILFLTCISVHRCLGVLRPLRSLRWGRARYARRVAGAVWVLVLACQAPVLYFVTTSARGGR
VTCHDTSAPELFSRFVAYSSVMLGLLFAVPFAVILVCYVLMARRLLKPAYGTSGGLPRAK
RKSVRTIAVVLAVFALCFLPFHVTRTLYYSFRSLDLSCHTLNAINMAYKVTRPLASANSC
LDPVLYFLAGQRLVRFARDAKPPTGPSPATPARRRLGLRRSDRTDMQRIEDVLGSSEDSR
RTESTPAGSENTKDIRL
|
|
|
|---|
| BDBM50205413 |
|---|
| n/a |
|---|
| Name | BDBM50205413 |
|---|
| Synonyms: | CHEMBL221326 | P(1),P(4)-bis(uridin-5'-yl) tetraphosphate | P1,P4-Bis(5'-uridyl) tetrahydrogen tetraphosphate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H26N4O23P4 |
|---|
| Mol. Mass. | 790.3071 |
|---|
| SMILES | O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)O[C@H]([C@@H]1O)n1ccc(=O)[nH]c1=O |r| |
|---|
| Structure | 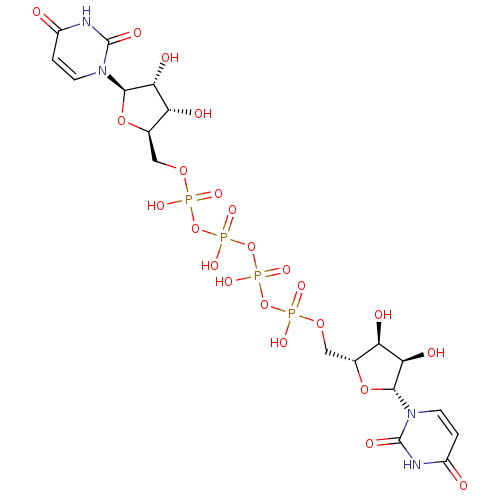
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yelovitch, S; Camden, J; Weisman, GA; Fischer, B Boranophosphate isoster controls P2Y-receptor subtype selectivity and metabolic stability of dinucleoside polyphosphate analogues. J Med Chem55:437-48 (2012) [PubMed] Article
Yelovitch, S; Camden, J; Weisman, GA; Fischer, B Boranophosphate isoster controls P2Y-receptor subtype selectivity and metabolic stability of dinucleoside polyphosphate analogues. J Med Chem55:437-48 (2012) [PubMed] Article